5 Important Pubs that Explore the Tumor Microenvironment
Exploration is in our DNA. As Homo Sapiens developed in the habitable regions of Africa, search of new food sources, climate changes, and mere curiosity pushed us beyond our confines to explore and conquer the planet.
In the beginning, we only had the stars for navigation, and our ability to know precisely where we were or where we were going was limited. Then, early explorers developed maps, compasses, and sextants, and our ability to know our location and plot our course improved tremendously. Today, we have tools like GPS, the Global Positioning System that allow us to know our exact location and surroundings down to the millimeter, and route our travel on land, sea, and sky.

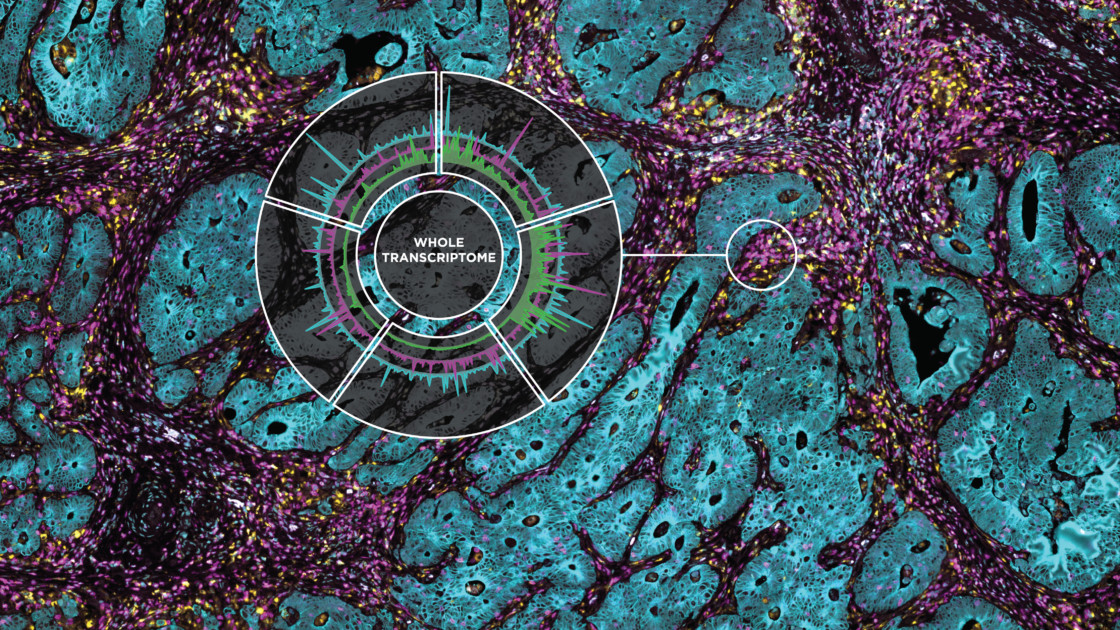
Similarly, our ability to map and characterize the tumor microenvironment has grown in similar ways – from early crude histology and single plex expression analyses, to genome-wide analysis, RNA sequencing, and multiplexed IHC. And today we finally have the tool to leap into the next biology revolution: DSP, the Digital Spatial Profiler that allows generating a whole tissue image at single-cell resolution and digital profiling data for 1,000’s of RNA or protein. And it’s awesome.
Indeed, while much has been learned about the tumor microenvironment, or TME, through bulk gene expression analysis and immunohistochemistry, researchers need to map what is happening and where it is occurring to develop effective treatments tailored to the individual’s cancer and TME. GeoMx Digital Spatial Profiling (DSP) helps researchers create a detailed map of the TME.
What is the Tumor Microenvironment?
Tumors need nutrients to grow and evolve, thus developing a complex and dynamic relationship with the surrounding microenvironment – blood vessels, immune cells, extracellular matrix, and fibroblasts – that influences its growth, invasion, and metastasis.
The Technology to Comprehend the Relationship between Tumor and Microenvironment
Here we review publications illustrating the impact DSP is having on immuno-oncology and translational research, starting with DSP itself. DSP is described in the Nature Biotechnology Letter “Multiplex digital spatial profiling of proteins and RNA in fixed tissue”[i]. This paper is rich in details about the techniques, samples, application, and validation of the technology. It is a must-read for those new to DSP or for those who are considering how to apply it to their own research.
The next four papers show applications of the DSP technology to address critical questions in immune-oncology. In these examples, spatial profiling is used to characterize fundamental aspects of tumor biology, tumor microenvironment, and the impacts of therapeutic intervention. They represent only a few of the many great publications that have resulted from DSP profiling.
Turning Cold Regions into Hot
Prostate cancer exists in a highly immunosuppressive environment, although tumor-associated lymphocytes are found outside the tumor margin. Localized, high-dose radiation brachytherapy (HDRBT) has been shown to “jump-start” the immune response.
In “High dose-rate brachytherapy of localized prostate cancer converts tumors from cold to hot”[ii], the authors investigated if HDRBT would overcome the immunosuppressive tumor microenvironment and enable immune cell infiltration past the tumor margin. In direct response to HDRBT, “cold” regions of immunosuppressive activity converted to “hot” regions of the immune response as characterized by a modified 16 gene tumor inflammation signature. And while that may have been previously indicated by bulk RNA sample analysis, it was DSP that elucidated the details of the cellular and molecular response. With this new information, researchers can now begin to assess patients’ responses to improve outcomes for those with prostate cancer.
Four Proteins to Predict Treatment Response
In the case of acute myeloid leukemia (AML), a small subset of patients form solid tumors and thus have a tumor microenvironment. These have been categorized into six immune subtypes and immunogenic profiles have been associated with therapeutic and prognostic implications for conventional chemotherapy. As described in “Immune landscapes predict chemotherapy resistance and immunotherapy response in acute myeloid leukemia”[iii], the team used DSP to characterize 31 immuno-oncology related proteins to subcategorize the TME into immune-infiltrated and immune-depleted subtypes.
Furthermore, four proteins stood out as candidates with the potential to predict responses to treatment based on patients’ pre-treatment samples. In particular, immune-infiltrated AML cases that also displayed higher expression of IFN-g correlated with a positive response to flotetuzumab treatment. These data strengthen the predictive value of the AML subtype to patient outcome, particularly in response to specific monoclonal antibody therapy.

Dissecting the Contribution of Suppressor Cells in Pancreatic Cancer
Developing a predictive model for pancreatic ductal adenocarcinoma (PDAC) treatment based on the immune phenotype of the tumor is the basis of “Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma”[iv].
PDAC has only a 10% five-year survival rate, so there is very little time to waste when selecting a treatment regimen. Using PDAC organoid/immune cell co-cultures, the group demonstrated that polymorphonuclear myeloid-derived suppressor cells (MDSC) contribute to the immunosuppressive tumor microenvironment. The tyrosine kinase inhibitor cabozantinib can deplete MDSCs and DSP showed an increase in gene expression associated with immune action against the tumor.
The next steps include a more retrospective analysis of patient responses in parallel with additional genomic and proteomic characterization of the pre-treated and treated samples. This is a great example of how pre-clinical models like immune cell co-culture in an organoid model can yield hope to patients with pancreatic cancer.
There is Still a Lot to Learn about Tumor Microenvironment
The final paper uses DSP to surveil cancer patients during a clinical trial and reminds us that there is still much to be learned about the TME. Tertiary Lymphoid Structures (TLS) are aggregates of lymphoid cells found across the spectrum of human cancers that are heterogeneous in composition and are thought to play an active role in anti-tumor immune activity. B cell association with TLS is thought to improve patient outcomes. There is much to learn about the association of B cells within the TLS, and its response to immune checkpoint blockade (ICB) therapy.
The team behind “B cells and tertiary lymphoid structures (TLS) promote immunotherapy response”[v] follows melanoma patients in a phase 2 clinical trial with nivolumab monotherapy or in conjunction with ipilimumab. DSP was used to characterize cancer patient samples prior to and during treatment, resulting in a better articulation of known biomarkers and identified new biomarkers of ICB responses. Patients with higher expression levels of B cell markers prior to and during treatment had a positive response to ICB treatment.
Understanding the physical structures and their associated signaling pathways within the TME are two avenues for concurrent research not just for melanoma treatments but to also understand the overall role of the TLS in the immune-oncology space.
DSP has practical applications for immuno-oncology research and its capabilities extend into translational research. It will continue to be a vital tool in the development of personalized medicine, providing cancer patients with more options and therapies based on the patient’s own unique cancer profile.
For Research Use Only. Not for use in diagnostic procedures.
[i] Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, Hoang M, Jung J, Liang Y, McKay-Fleisch J, Nguyen K, Norgaard Z, Sorg K, Sprague I, Warren C, Warren S, Webster PJ, Zhou Z, Zollinger DR, Dunaway DL, Mills GB, Beechem JM. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat Biotechnol. 2020 May;38(5):586-599. doi: 10.1038/s41587-020-0472-9. Epub 2020 May 11. PMID: 32393914.
[ii] Keam SP, Halse H, Nguyen T, Wang M, Van Kooten Losio N, Mitchell C, Caramia F, Byrne DJ, Haupt S, Ryland G, Darcy PK, Sandhu S, Blombery P, Haupt Y, Williams SG, Neeson PJ. High dose-rate brachytherapy of localized prostate cancer converts tumors from cold to hot. J Immunother Cancer. 2020 Jun;8(1):e000792. doi: 10.1136/jitc-2020-000792. PMID: 32581061; PMCID: PMC7319782.
[iii] Vadakekolathu J, Minden MD, Hood T, Church SE, Reeder S, Altmann H, Sullivan AH, Viboch EJ, Patel T, Ibrahimova N, Warren SE, Arruda A, Liang Y, Smith TH, Foulds GA, Bailey MD, Gowen-MacDonald J, Muth J, Schmitz M, Cesano A, Pockley AG, Valk PJM, Löwenberg B, Bornhäuser M, Tasian SK, Rettig MP, Davidson-Moncada JK, DiPersio JF, Rutella S. Immune landscapes predict chemotherapy resistance and immunotherapy response in acute myeloid leukemia. Sci Transl Med. 2020 Jun 3;12(546):eaaz0463. doi: 10.1126/scitranslmed.aaz0463. PMID: 32493790; PMCID: PMC7427158.
[iv] Holokai L, Chakrabarti J, Lundy J, Croagh D, Adhikary P, Richards SS, Woodson C, Steele N, Kuester R, Scott A, Khreiss M, Frankel T, Merchant J, Jenkins BJ, Wang J, Shroff RT, Ahmad SA, Zavros Y. Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2020 Dec 17;12(12):3816. doi: 10.3390/cancers12123816. PMID: 33348809; PMCID: PMC7766822.
[v] Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020 Jan;577(7791):549-555. doi: 10.1038/s41586-019-1922-8. Epub 2020 Jan 15. PMID: 31942075.