Common questions in molecular biology: What are the advantages of DNA barcoding?
DNA barcoding is a method of identification based on DNA sequences made feasible by available genomic sequence data. Initially developed as a tool for rapid species identification (Hebert), DNA barcodes consist of a standardized short sequence of DNA (400–800 bp) that is identifiable by its specificity to the application at hand. This specificity of sequence is one of several advantages of DNA barcoding.
Specificity
DNA barcodes, in principle, can be generated for every species on the planet (Kress), especially considering the number of complete genomes that have been sequenced. In fact, biologists concerned with taxonomy are in the process of compiling a massive online digital library of species-specific barcodes for classifying unidentified specimens (Kress).
DNA barcoding allows biologists in the fields of evolution, ecology, and zoology to categorize unidentified specimens efficiently and reliably.
DNA barcoding allows biologists in the fields of evolution, ecology, and zoology to categorize unidentified specimens efficiently and reliably as either known or novel based on the information of only one or a few gene regions (Kress). Specificity also makes possible the use of DNA barcodes in high‐throughput sequencing (HTS) for simultaneous high‐throughput identification of DNA of different origins from complex multi‐taxa samples (Raclariu).
Specificity is also an advantage for engineered barcodes through the design of a “common specification that facilitates resolution of ambiguities” (Parameswaran). In other words, the sequence of DNA barcodes can be tailored for nearly any use imaginable.
Inherited
Since DNA barcodes are made of the genetic material of all living organisms, any organism carrying one will pass it along to its offspring, whether naturally occurring or engineered. Inherited DNA barcodes can therefore be tracked through generations, overcoming previous challenges of observing changes in clonal populations of cells over time.
DNA barcodes make it possible to identify and track many thousands of cell lineages in fields such as evolution, development, and cancer.
Previously a slow, labor-intensive process, DNA barcodes used as unique genetic markers in combination with modern genetic engineering and sequencing technologies allows high-throughput lineage tracking over multiple generations (Johnson). DNA barcodes make it possible to identify and track many thousands of cell lineages carrying different barcodes in fields such as evolution, development, and cancer (Johnson).
Cell lineage studies can be divided into two types, retrospective and prospective. Retrospective studies typically examine organismal development, inferring the lineage of a cell population based on variations observed in naturally occurring DNA barcodes. Prospective studies, in contrast, introduce random DNA barcodes into an organism for the purpose of observing future changes. The diversity of the barcodes used in prospective studies is usually generated in vitro before being integrated into the organism’s genome. Other methods have also been developed using a targeted-mutagenesis module for integration into the organism, generating barcode diversity at the in vivo locus (Johnson).
Manipulable and adaptable
Another advantage of DNA barcodes is their manipulability and adaptability to almost any molecular application. Each barcode (or tag) is identified by its unique sequence, enabling researchers to use them wherever they are needed. Each barcode’s unique sequence means it can be identified by PCR, sequencing, or direct hybridization. Direct hybridization protocols identify incorporated tags by hybridization to labelled purified plasmids or to purified tag DNA, greatly increasing the sensitivity of the assay and allowing the use of non-radioactive detection methods, such as digoxigenin, biotin, or fluorophores. The reliability of the barcode signals can be increased by incorporation of longer tags or multiple tags (Mazurkiewicz).
Each barcode’s unique sequence means it can be identified by PCR, sequencing, or direct hybridization.
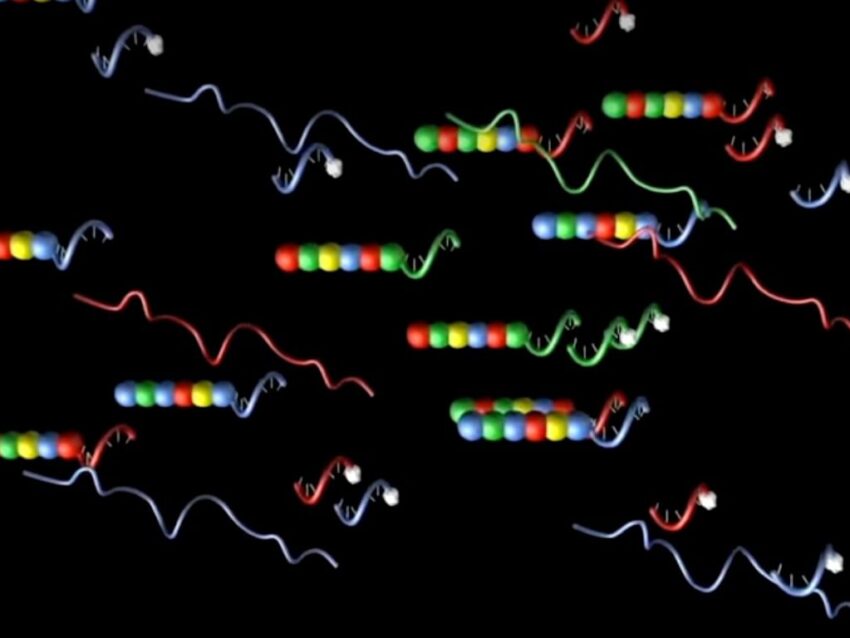
Further adaptations of the DNA barcoding concept has been done by companies such as NanoString for use in studying gene expression. More accurately termed molecular barcoding, NanoString’s technology used in its nCounter® Pro Analysis System incorporates both DNA and RNA barcode segments in its probes, along with fluorescent molecules.
Using a simple, reproducible hybridization protocol, NanoString’s molecular barcodes uniquely identify subsets of genes using panels of barcode-labeled probes for high-throughput multiplex screening of transcriptomes (Geiss). The RNA of each probe consists of multiple RNA segments, each labeled with a specific fluorophore. The RNA barcode is created by arranging the differently colored RNA segments in a specific linear order to generate a unique code for each gene of interest. These fluorescent RNA barcodes are then annealed to a single-stranded DNA molecule, known as the backbone, that is ligated to target-specific DNA sequence (Geiss). This reporter probe is one half of a probe pair that works in concert with a capture probe generated by fusing gene-specific sequence adjacent to that of the reporter probe to a series of 3’ repeats (Geiss).

The optimized hybridization protocol used with this probe system, when annealed to the target RNA, creates a tripartite structure of reporter probe, capture probe, and target molecule. Each unique fluorescent barcode, in addition to identifying the gene, also directly counts each transcript without the need for enzymatic or amplification steps, producing reliable, highly sensitive gene expression profiling data, even on low copy number transcripts (Geiss).
References
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008 Mar;26(3):317-25. doi: 10.1038/nbt1385. Epub 2008 Feb 17. PMID: 18278033.
Johnson MS, Venkataram S, Kryazhimskiy S. Best Practices in Designing, Sequencing, and Identifying Random DNA Barcodes. J Mol Evol. 2023 Jan 18. doi: 10.1007/s00239-022-10083-z. Epub ahead of print. PMID: 36651964.
Mazurkiewicz P, Tang CM, Boone C, Holden DW. Signature-tagged mutagenesis: barcoding mutants for genome-wide screens. Nat Rev Genet. 2006 Dec;7(12):929-39. doi: 10.1038/nrg1984. PMID: 17139324.
Parameswaran P, Jalili R, Tao L, et al. A pyrosequencing-tailored nucleotide barcode design unveils opportunities for large-scale sample multiplexing. Nucleic Acids Res. 2007;35(19):e130. doi:10.1093/nar/gkm760
Raclariu AC, Heinrich M, Ichim MC, de Boer H. Benefits and Limitations of DNA Barcoding and Metabarcoding in Herbal Product Authentication. Phytochem Anal. 2018;29(2):123-128. doi:10.1002/pca.2732

Related Content