Utilizing Nonhuman Primate Models and High-Dimensional Spatial Approaches to Understand HIV Persistence
View On Demand
Abstract
HIV cannot be eradicated by antiretroviral therapies (ART) alone. Although lifelong suppression of HIV replication with ART seems possible, side effects, stigma, resistance, need for lifelong adherence, and cost all contribute to the necessity of finding an HIV ‘Cure’. The major obstacle to curing HIV is the persistence of long-lived viral reservoirs that remain throughout the body despite extended suppressive ART, which gives rise to recrudescent infection when ART is stopped. In addition to the highly studied CD4+ T cell reservoir (and other infected cellular reservoirs) that harbor integrated, replication-competent provirus within their cellular DNA, an additional less studied HIV reservoir that is resistant to clearance by host immune mechanisms is follicular dendritic cells (FDC). FDCs remain uninfected but can bind (via complement receptors) and retain intact infectious virions for long periods of time in the form of immune complexes and can infect CD4+ TFH cells within B cell follicles. A comprehensive understanding of all HIV reservoirs that have the potential to reignite systemic infection after ART is discontinued is fundamental before an HIV cure can be fully realized.
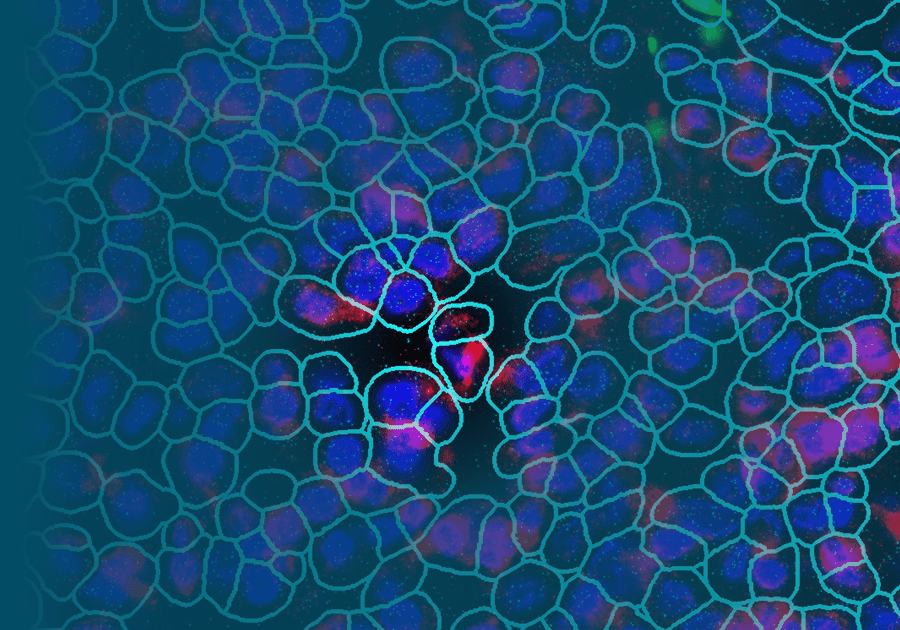
Here, I will discuss data using a Simian Immunodeficiency Virus (SIV) infected nonhuman primate (NHP) rhesus macaque (RM) model and a novel biologic therapy that blocks CD21 (complement receptor 21) aimed at preventing and eliminating the SIV-FDC reservoir. I will show data using an array of spatial profiling approaches in FFPE lymph nodes, including RNAscope in situ hybridization and NanoString® GeoMx® and CosMx™ Spatial Molecular Imager (SMI) platforms, to characterize the impact of anti-CD21 treatment on FDC-SIV trapping and the impact to the transcriptional landscape of cells within the B cell follicle tissue microenvironment and cellular immune neighborhoods surrounding infected cells. Comprehensive spatial profiling of tissue samples provides insight beyond what is possible from bulk analyses and demonstrates the impact of therapeutic intervention in the context of tissue microenvironments.
These results improve our understanding of the unique FDC-HIV reservoir and provide an effective treatment approach for targeting this viral reservoir as part of a combinatorial HIV cure strategy.
Speaker

Jacob D. Estes, PhD
Vaccine and Gene Therapy Institute, Oregon Health and Science University
Affiliations: Vaccine and Gene Therapy Institute, Oregon Health and Science University, Beaverton, OR, USA Division of Pathobiology and Immunology, Oregon Health and Science University, Beaverton, OR, USA Adjunct Professor, School of Health and Biomedical Sciences, College of Science, Engineering and Health, RMIT University, Melbourne, Australia Honorary Professor of Immunopathology, Faculty of Health, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark

