Alzheimer’s Disease Pathology: Fear, Confusion, and the Search for a Cure
Alzheimer’s disease pathology: a short sentence loaded with fear, confusion, and incredulity.
The word “Alzheimer’s” first appeared in a German psychiatry handbook called “General Psychiatry” on July 15, 1910. This chronic neurodegenerative disease got its name from the work of a German psychiatrist, Alois Alzheimer, who conducted an autopsy on his patient who had suffered loss of memory, disorientation, and severe dementia.
The autopsy of the brain revealed to him two distinctive characteristics of the disease: tangled clumps of nerve fibers and patches of disintegrated nerve cell branches. It is now well known that the pathogenesis of Alzheimer’s disease (AD) is associated with the development of amyloid-β (Aβ) plaques in the brain and neurofibrillary tangles inside the neurons.
Despite extensive research, the cause of Alzheimer’s disease is not yet clear. Currently, there are two main hypotheses proposed for the cause. The Amyloid hypothesis suggests that the degradation of Aβ, derived from the proteolytic cleavage of amyloid precursor protein (APP), decreases with age or pathological conditions. This leads to the accumulation of Aβ peptides (Aβ40 and Aβ42), an increasing ratio of Aβ42/Aβ40, which induces Aβ amyloid fibril formation and tau (a neural protein) pathology induction, and, consequently, leads to neuronal cell death and neurodegeneration.
The Cholinergic hypothesis suggests that AD is due to the reduction in the levels of the neurotransmitter acetylcholine which is synthesized by cholinergic neurons. Degeneration of these cholinergic neurons is found to take place in AD. Studies have demonstrated that cholinergic synaptic loss and amyloid fibril formation are related to Aβ oligomer neurotoxicity, and to interaction between acetylcholine and Aβ peptide. Several studies indicate that AD is a multifactorial disease resulting from a complex crosstalk between different pathophysiological processes.
Drug development and Alzheimer’s Disease
The search for a drug to cure AD has been elusive. To date, only five medical treatments have been approved for AD. But these drugs act to control the symptoms rather than alter the course of the disease.
For the past 20 years, knowledge of Aβ plaques has been the focus of most Alzheimer’s drug development. The accumulation of Aβ protein is thought to be the initiating event of AD that triggers a series of downstream processes that leads to synaptic damage and neuronal loss. Recently, the Food and Drug Administration (FDA) gave conditional approval to the drug Aducanumab based primarily on the medication’s ability to remove amyloid from the brain. Data from phase III trials showed that the drug effectively removes plaques by 55% to 60%. However, it is not very clear if the removal of plaques is beneficial to AD patients.
Today, there is an increasing diversity of targets and corresponding therapeutic mechanisms of drugs in the AD pipeline. To coordinate efforts, planning, and resources on a global scale, an analysis tool known as the Common Alzheimer’s Disease Research Ontology (CADRO) has been developed by the National Institute on Aging and the Alzheimer’s Association.
CADRO classifies drugs into two categories: those meant for symptomatic treatments, and those designed for disease-modifying treatments. Currently, there are 126 agents in 152 trials of treatments for AD, as of the index date of January 5, 2021. Many of the drugs in trials today (82.5%) target the underlying biology of AD, with the intent of disease modification.
Based on the cholinergic hypothesis, one of many therapeutic strategies explored has been to inhibit the degradation of the neurotransmitter acetylcholine. Tacrine was the first FDA-approved cholinesterase inhibitor drug, but it exited the market immediately after its introduction due to a high incidence of side effects and a lack of benefits.
Currently, several cholinesterase inhibitors such as Donepezil, Rivastigmine, and Galantamine are being used for symptomatic treatment. The effects of these drugs on cognition and function are generally modest and response rates are variable. A few examples of new drugs using the cholinergic hypothesis approach for cognitive enhancement are in the pipeline: AD-35, Octo-hydro-amino acridine succinate and the Nicotine transdermal patch.
Excitatory glutamatergic neurotransmission via N-methyl-d-aspartate receptor (NMDAR) is critical for synaptic plasticity and survival of neurons. However, excessive NMDAR activity causes excitotoxicity and promotes cell death. Therefore, it is believed to have a dominant role in the pathophysiology of AD. Several NMDAR noncompetitive antagonists have been developed and entered in clinical trials. However, most of them have failed due to low efficacy and side effects.
Memantine is the only approved drug that uncompetitively blocks the NMDA receptor and is thought to be neuroprotective in function. It is generally well-tolerated, with fewer side effects than cholinesterase inhibitors, although some side effects do occur. A few new drugs in trials are AVP-786 for managing neuropsychiatric symptoms and SAGE-718 for cognitive enhancement.
The Alzheimer’s tau protein is a promising target for future treatments as it seems to be better correlated to the severity of cognitive decline than Aβ. Aggregation of Aβ causes hyperphosphorylation of tau, which then leads to the formation of tau aggregates. These tau aggregates form twisted paired helical filaments intracellularly known as neurofibrillary tangles.
Currently, most anti-tau agents in clinical trials are immunotherapies and are in the early stages of clinical research. So far, four monoclonal anti-tau antibodies Gosuranemab, Tilavonemab, Semorinemab, and Zagotenemab, and one anti-tau vaccine (AADvac1) have reached phase II clinical trials.

The future of Alzheimer’s disease research: spatial transcriptomics
AD drug development has a high rate of failure. In many cases, blood-brain barrier penetration of the drug, dosage, target engagement by the drug, or rigorous interrogation of early-stage data has not been pursued. Further, analysis of predictors of success in drug development programs shows that agents linked to genetically defined targets have a greater chance of being advanced from one phase to the next. In such situations, transgenic mouse models can add to the genetic evidence for a target.
Using tools such as NanoString’s GeoMx® Mouse Whole Transcriptome Atlas (WTA), one can spatially resolve gene expression in tissues from AD patients to help prioritize drug candidates as well as support target validation. As an example, Dr. Frances Edwards’s lab at the University College of London used a mouse model of AD to study how microglia gene expression correlates with amyloid-beta buildup. Using the Mouse WTA, microglia gene expression was investigated in relation to distance from Aβ plaques in a cell-specific manner. It will be interesting to see how this data could help researchers understand the role of microglia in AD initiation and progression which may, in turn, help design targeted therapeutics.
Mounting evidence suggests that the pathological changes associated with AD begin to occur several years before the emergence of the clinical syndrome. Many of the drugs developed could be beneficial at an early stage before the neurodegenerative process sets in.
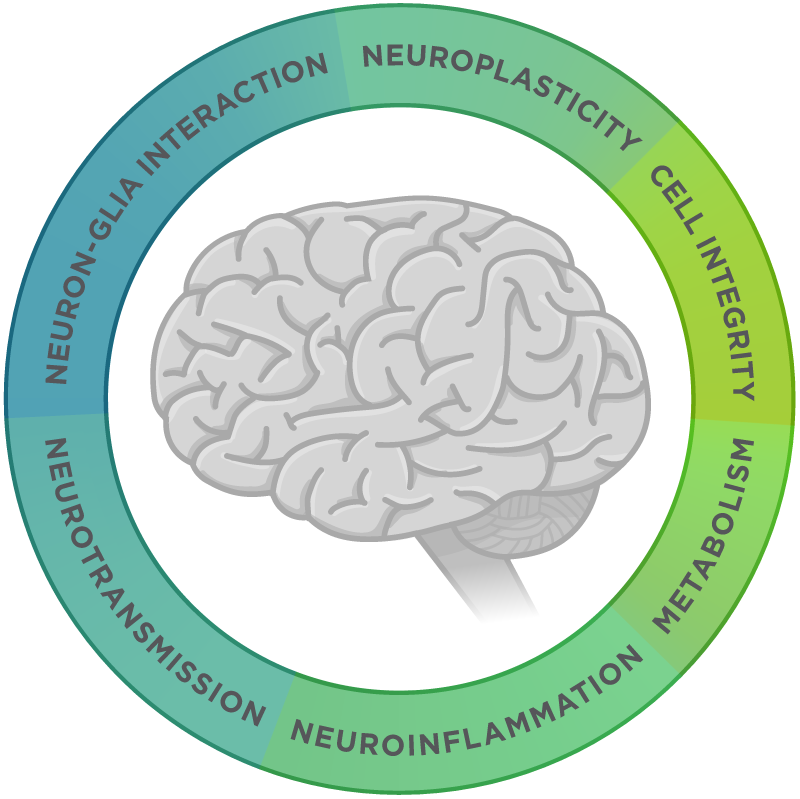
Towards this aim, the application of an expression profiling technology to AD could help identify Alzheimer’s biomarkers for early detection. NanoString’s nCounter® Analysis System can quantify differential gene expression changes for up to 800 genes for a given sample without any enzymatic sequencing steps. For example, the nCounter Neuropathology Panel is designed to include genes involved in: neurotransmission, neuron-glia interaction, neuroplasticity, cell structure integrity, neuroinflammation, and metabolism. This tool therefore could help dissect changes in gene expressions during the progression of AD.
Finally, next-generation biotherapeutics include immune/stem cell, gene, and nucleotide therapies. Currently, there are nine trials of cell therapies in AD, one oligonucleotide targeting tau expression, and several epigenetic modulators. With these advancements, it becomes a necessity during trials to understand how changes in gene expression rapidly drive the efficacy of these agents, and spatial transcriptomics is rapidly becoming the solution.
For Research Use Only. Not for use in diagnostic procedures.