Spatial Multiomics Identifies New Actionable Therapeutic Targets
Pancreatic cancer represents 3% of the overall cancers in America and yet is responsible for 7% of all cancer deaths. The five-year relative survival rate – the cancer-related death rate compared to the death rate of the rest of the population – is 42% when the cancer is localized but goes down to only 3% when it spreads. The overall survival rate is about 11%. As a rare cancer, it is one of the deadliest.
There is currently no screen for pancreatic cancer; therefore, it is often diagnosed late. The earlier a tumor is detected, the better the outcome is, but only 10% of patients are lucky enough for it to be detected early enough when it can be removed by surgical ablation. Most of the time, by the time the symptoms are present, the tumor has spread. At this point, because the pancreas is in a highly vascularized part of the body and near to major blood vessels that come off the aorta, it is inoperable.
Pancreatic cancer is also not responsive to treatment, whether it’s classic chemo and radiation therapy or targeted therapy. Understanding why it escapes treatment is critical for effective diagnosis and treatment.
In this two-part blog miniseries, we will explore some of the reasons why pancreatic cancer and, in particular pancreatic ductal adenocarcinoma, is so deadly and what a group of Harvard scientists has discovered using spatial multiomics that may tip the scales in our favor.
What is Pancreatic Cancer, and is it Curable?
The pancreas is a glandular organ made of two kinds of glands. This distinction is important as these two glands give rise to different types of pancreatic cancer.
- Endocrine glands–like the Islets of Langerhans–secrete hormones such as insulin or glucagon into the bloodstream. A tumor arising in this part of the organ is called a pancreatic neuroendocrine tumor.
- Exocrine cells–the acinar cells—secrete digestive enzymes into the duodenum. A tumor in this part of the organ looks like a duct under the microscope; therefore, it is called pancreatic ductal adenocarcinoma (PDAC).
PDAC is the most prevalent pancreatic malignancy, accounting for over 90% of pancreatic cancers. Treatment is elusive. Even immunotherapies such as immune checkpoint inhibitors have limited success, presumably because of an immune suppressive and desmoplastic (fibrotic growths around the cancer) microenvironment or for a low mutational burden (resulting in a lower number of neoantigens).
Furthermore, the acinar cells retain a high degree of plasticity that appears during tissue damage that can occur during prolonged inflammation and/or stress when, in a process known as acinar-to-ductal metaplasia (ADM), these cells acquire a progenitor-like characteristic and differentiate into a ductal-like phenotype. The progenitor cell-like phenotype exposes these cells to pro-oncogenic mutations as the frequent regenerations make them susceptible to reactivation of pro-oncogenic genes and turn them into pancreatic intra-epithelial neoplasia (PanIN), the initial stage of PDAC. Simply put, pancreatic cells, in their response to repeated or chronic inflammation meant to protect the organ, develop mutant forms of genes controlling cell growth and proliferation, promoting cancer.
The Key Characteristics of PDAC
PDAC has certain characteristics that make it not only deadly but also difficult to study.
Tumors in the pancreas are highly fibrous due to an extensive desmoplastic stroma originated by fibroblasts – cancer-associated fibroblasts (CAFs) – that deposit large amounts of extracellular matrix in the microenvironment; this condition is also a strong factor of hypo-vascularization. Therefore, another PDAC characteristic is hypoxia.
These conditions create an infiltration barrier to T cells as well as myeloid cells that tend to accumulate in the PDAC tumor microenvironment. These myeloid cells acquire a suppressive phenotype and further block T cells from entering the microenvironment. Myeloid presence in PDAC is a marker of poor prognosis.
PDAC is also highly resistant to any therapy. The mechanisms behind this are multifactorial but still poorly understood. Where single-agent therapies have failed, combination therapy may offer better results. Deciphering the impact of treatment on residual cells to individuate weaknesses to use in therapies is paramount.
Bulk RNA profiling has helped in stratifying PDAC into two subtypes:
- Classic/epithelial
- Basal-like/squamous, which is associated with poorer response and survival.
RNA Sequencing (RNA-seq) has uncovered other possible subtypes associated with microenvironment features, but they do not have an impact on patient response.
Single-cell RNA seq (scRNA-seq) could reveal deeper diversity in the TME, but the dense fibrous tissue – i.e., desmoplastic tissue – surrounding the area makes it challenging. An alternative is single nucleus sequencing (snRNA-seq), a relatively novel technique that sequences isolated nuclei instead of single cells; the idea is that nuclei are easy to isolate and preserve, ideal in situations like PDAC where cells are hard to isolate.

Integrating Spatial Multiomics and Spatial Analysis to Understand PDAC
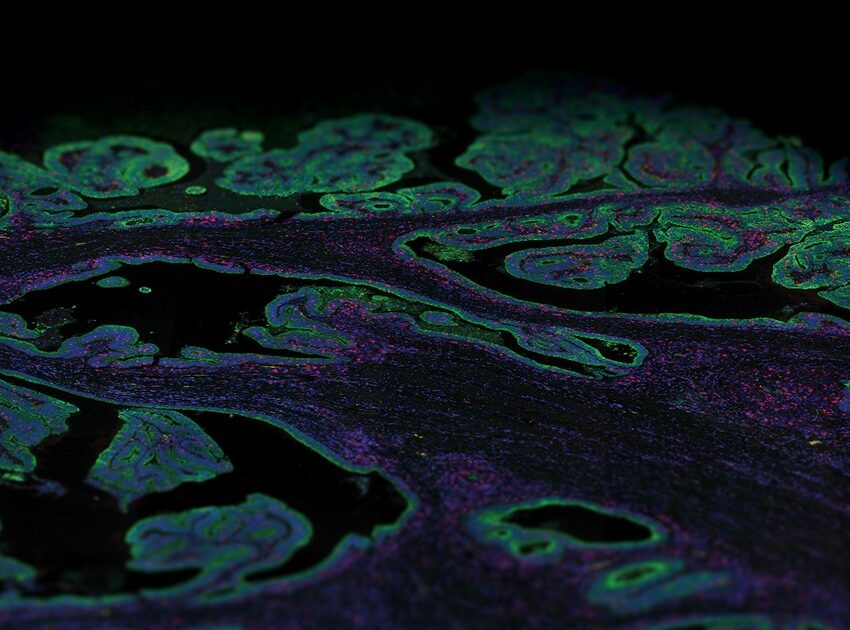
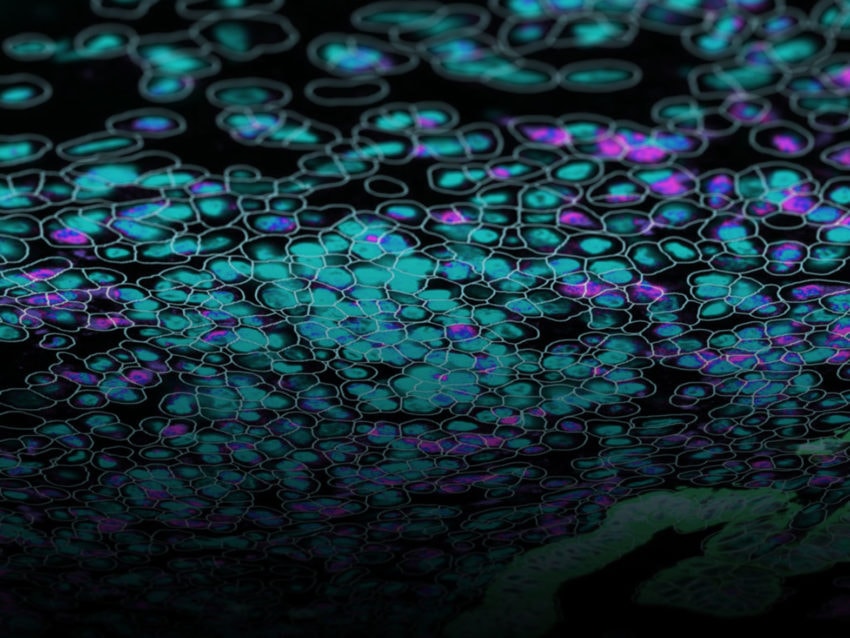
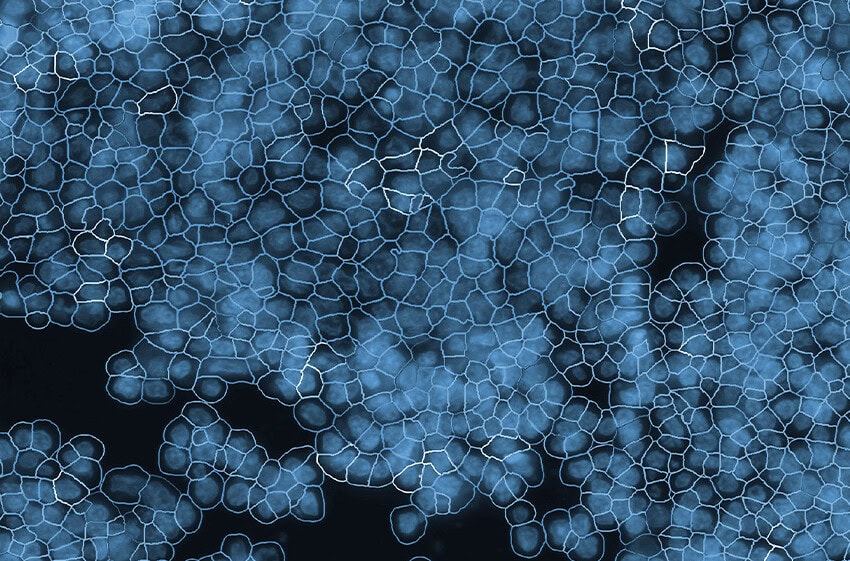
Will Hwang and colleagues, in an extraordinary team effort led by the Center for Systems Biology at Harvard Medical School, in collaboration with NanoString, used single nucleus RNA seq, the spatial multiomics platform GeoMx® Digital Spatial Profiler with the Whole Transcriptome Atlas (WTA) to profile tumor samples from a cohort of pancreatic cancer patients before or after neoadjuvant therapy.
The authors uncovered a molecular program that develops after treatment in the residual cancer cells and therefore renders them resistant to treatment. This is a major finding, as this program can be a potential target for new therapies.
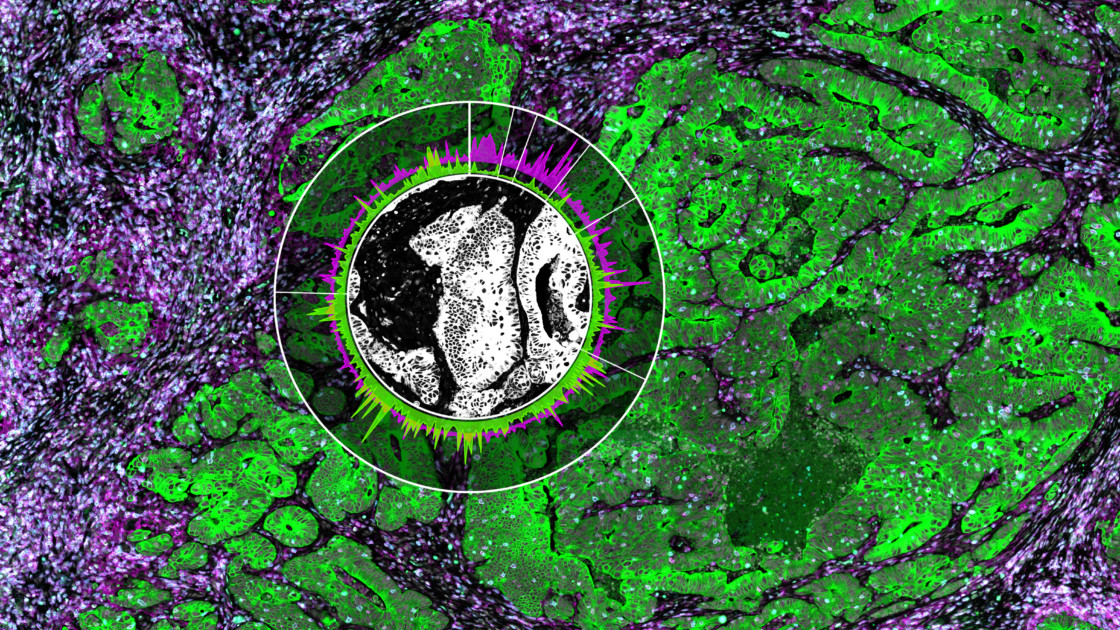
Moreover, by integrating these cell type signatures and expression profiles with the GeoMx Whole Transcriptome Atlas, the authors identified several multicellular communities, including one composed of the newly discovered molecular program and CD8 T cells. This community was associated with treatment resistance and poor prognosis.
GeoMx, by spatially localizing the communities’ positions, revealed that these programs can co-exist on the same tissue and patient, further demonstrating that therapies will need to target different programs and subtypes within the same cancer.
GeoMx DSP also revealed the spatial location of ligand/receptor interactions enhanced only in post-treatment residual disease, thus expanding the potential for new neoadjuvant and adjuvant therapies.
In part two of this blog post miniseries, we will go into more details about this seminal paper and explore how Dr. Hwang and colleagues uncovered new multicellular dynamics associated with PDAC.
Bibliography
Hwang, W.L., Jagadeesh, K.A., Guo, J.A. et al. Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment. Nat Genet 54, 1178–1191 (2022). https://doi.org/10.1038/s41588-022-01134-8
Moffitt, R., Marayati, R., Flate, E. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 47, 1168–1178 (2015). https://doi.org/10.1038/ng.3398
Truong, L.-H.; Pauklin, S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers 2021, 13, 5028. https://doi.org/10.3390/cancers13195028
Dylan Kotliar, Adrian Veres, M Aurel Nagy, Shervin Tabrizi, Eran Hodis, Douglas A Melton, Pardis C Sabeti (2019) Identifying gene expression programs of cell-type identity and cellular activity with single-cell RNA-Seq eLife 8:e43803 https://doi.org/10.7554/eLife.43803
For Research Use Only. Not for use in diagnostic procedures.