Common questions in molecular biology: What are the most common biomarkers?
If you stopped someone on the street and asked them to give you an example of a common biomarker, you will more than likely be met with silence.
However, if you reframe the question and ask them to tell you what happens when they visit the doctor’s office for a routine physical, you’ll get a ready response. The standard measurements that are taken at the doctor’s office every time you go — weight, blood pressure, pulse — are all examples of biomarkers. Routine blood draws, whether they include a complete blood count, assessment of hormone levels in the blood, or genetic testing, are also assessing biomarkers.
Since each of these examples is a quantifiable, objective, and reliable metric that indicates something about your overall health, they are considered biomarkers. In this Common questions in molecular biology blog post, I explore the features of biomarkers, give examples of several common kinds of biomarkers and describe how they are used clinically, and close with the future discovery of new biomarkers through single-cell sequencing.
What are distinguishing features of biomarkers?
For something to be considered a biological marker or biomarker, there are several criteria that must be met. First, it must be an objective measure. A patient stating “they don’t feel good” is very subjective: what constitutes feeling sick to one person may not for another. By the same measure, different clinicians may make different professional judgements regarding how sick someone may be. There are also risks of confirmation bias by either party. Consequently, there is a need to collect objective information about a patient that can be compared to a standard.
Related to objectivity, a biomarker must be quantifiable. In order to compare it to others, we need to be able to quantify it. This could refer to the amount of gene expression or some other amount, such as how much one weighs. How this biomarker is quantified must also be reproducible in a reliable manner. For example, there should be minimal variability if different people took the same person’s blood pressure in quick succession.
Biomarkers much be objective, quantifiable, reproducible, and indicate something of use.
Biomarkers must also tell us about someone’s health. The key is that they are “marking” something. For instance, we can draw a parallel between boating and the presence and location of buoys. One would not go through the process of placing a buoy if there wasn’t a compelling reason to mark the area — to warn of an underwater obstacle, for example, or mark allowed boating lanes in a wildlife preserve. An arbitrarily-placed buoys would have no value; neither would a biomarker that doesn’t tell us anything useful.
What are common biomarkers used by doctors or researchers?
There are a wide variety of categories of biomarkers, and methods for categorizing biomarkers. Biomarkers are typically grouped either by type (e.g., whether what is being measured is the whole body, an organ, or at the cell or subcellular levels) or by uses.
Biomarkers have a wide variety of uses in clinical or research settings. For example, assessment of biomarkers is useful to determine if a treatment modality is successful. One could assess if treatment with a new drug designed to lower blood pressure actually does.
When evaluating a cancer treatment, researchers could evaluate biomarkers to decide on the best course of treatment. These are called predictive biomarkers. For example, a researcher may choose to look and see if cancer cells express the Epidermal Growth Factor Receptor (EGFR). This could dictate if treatment with an EGFR inhibitor is warranted or not.
Also within the context of cancer treatment, a clinician or researcher could use biomarkers such as levels of carcinoembryonic antigen or CEA. CEA levels are used to monitor cancer progression. Therefore, one could determine if the treatment is effective by monitoring cancer progression or regression via CEA levels.
Diagnostic, monitoring, predictive, prognostic, and susceptibility biomarkers are all commonly used by clinicians to improve care.
Clinicians also use prognostic and susceptibility biomarkers. Although in principle these biomarkers sound the same, they actually have different uses. Prognostic biomarkers are used to predict how bad a person’s disease will affect them (e.g., making a prognosis). For example, when one is diagnosed with prostate cancer, doctors will assess their levels of Prostate Specific Antigen (PSA). PSA levels correspond to a patient’s one- and five-year survival rates. Somewhat similarly, women diagnosed with triple negative breast cancer (meaning, the cancer cells do not express the estrogen or progesterone receptors, nor human epidermal growth factor receptor 2) typically have a poorer prognosis than other types of breast cancer.
A susceptibility biomarker indicates the likelihood that someone will develop a disease. These are assessed prior to the development of any illness. This could include if a person has certain gene mutations. For example, one commercially available test screens for susceptibility to eleven different types of cancer by examining 48 known gene variants associated with the development of each of these forms of cancer. Knowing one’s risk factors can lead to different life choices and/or increased or earlier cancer screenings.
What is the future of biomarkers?
Although the use of standard biomarkers to gauge health are unlikely to go away, the future of biomarkers firmly falls in the molecular realm. For example, our understanding of cancer is changing from focusing on the tissue of origin to instead the molecular signature of tumors reflected in various biomarkers. Therefore, treatment decisions will rely heavily on these biomarkers.

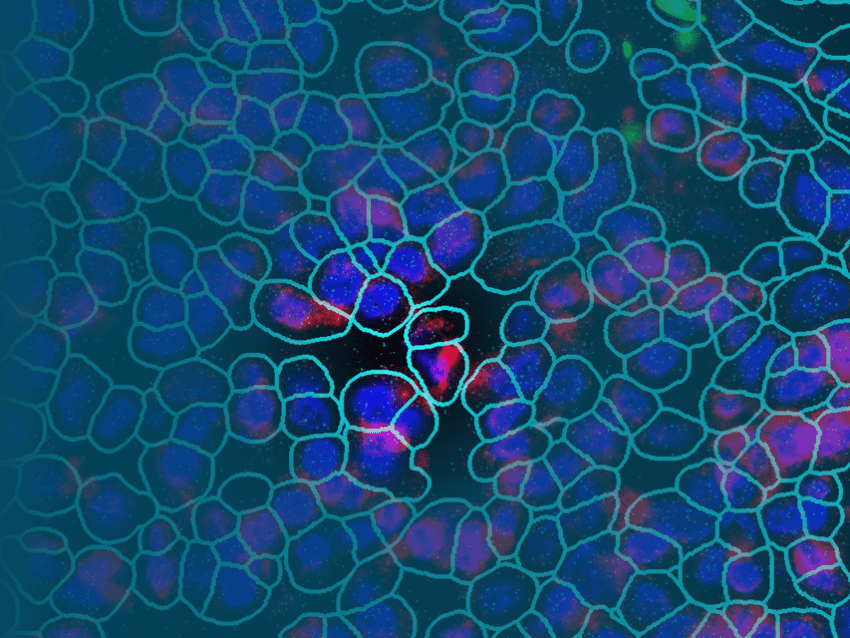
One exciting recent development in biomarker discovery is the advent of technologies that facilitate spatially resolved single-cell sequencing. One such technology is NanoString’s CosMx™ Spatial Molecular Imager. Although researchers have used single cell RNA seq, a next-generation based technology, to identify biomarkers, single-cell sequencing adds an additional layer of complexity. Not only can researchers look at genomic information, but gene expression alongside proteomic data, all within the spatial context. Together, this provides critical information to enable a better understanding of individual cells and how they function within their specific tissue context.
Molecular biomarkers are increasingly common
There are many common biomarkers, and assessment of one’s biomarkers is a normal part of any visit to the doctor’s office. With the advent of new technologies, including spatially resolved single-cell sequencing using NanoString’s CosMx SMI, we can look forward to an exciting future with more specific and informative biomarkers.
The CosMx™ SMI and decoder probes are not offered and/or delivered to the Federal Republic of Germany for use in the Federal Republic of Germany for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof in a method used in fluorescence in situ hybridization for detecting a plurality of analytes in a sample without the consent of the President and Fellows of Harvard College (Harvard Corporation) as owner of the German part of EP 2 794 928 B1. The use for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof is prohibited without the consent of the President and Fellows of Harvard College (Harvard Corporation).