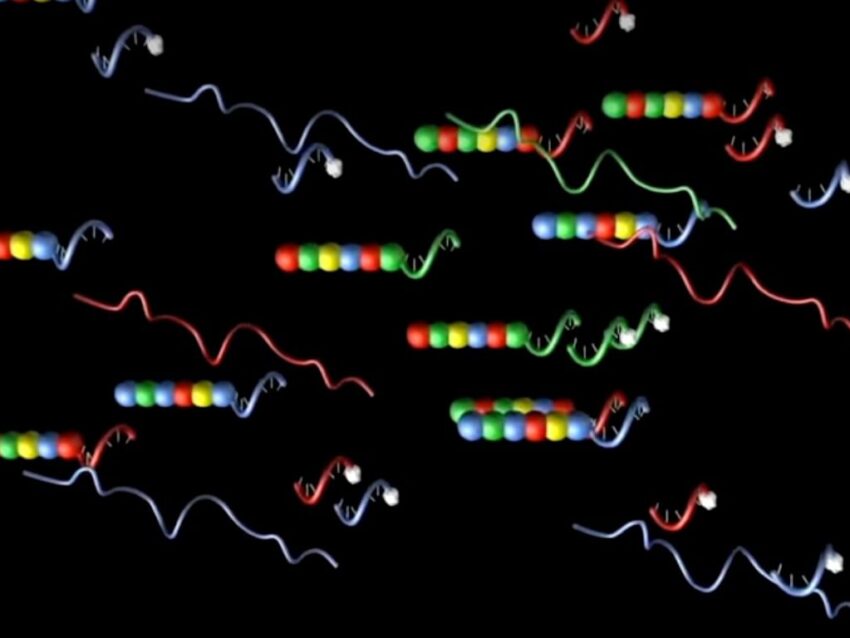
Common questions in molecular biology: What is an example of an oligonucleotide?
Oligonucleotides, short polymers of the nucleotide building blocks of nucleic acids such as DNA or RNA, come in many forms. In nature, oligonucleotides exist mainly as small non-coding RNAs such as microRNAs (miRNAs), but can also be chemically synthesized DNA or RNA molecules. Whether naturally occurring or synthetic, oligonucleotides anneal to complementary targets such as genomic DNA or the various cellular RNAs. Relatively small (typically 12–25 base pairs), oligonucleotides (also known as oligomers or oligos) are used in an extensive range of molecular genetics applications and may be chemically modified or unmodified.
Oligonucleotide structure
All oligonucleotide monomers consist of three components: a nucleotide or base (either a purine or a pyrimidine), a pentose five-carbon sugar (either ribose for RNA or deoxyribose for DNA), and a phosphate group. Adjacent monomers (individual nucleotides) are connected via their phosphate groups to form, along with the sugar moieties, the backbone of the nucleic acid. Every nucleic acid strand has a directionality running from the 5′-end to 3′-end.
In double-stranded DNA, the two strands are oriented in opposite directions to allow complementary base pairing between nucleotides. The nucleotides fundamental to all living organisms are divided into purines — adenine (A) and guanine (G)- and pyrimidines — cytosine (C), thymine (T; in DNA), and uracil (U; in RNA). Specific hydrogen-bonding, known as complementarity, occurs between adenine and either thymine or uracil, while guanine and cytosine bond with each other. Nucleotides may be grouped into mononucleotides, dinucleotides, oligonucleotides, and polynucleotides.
Oligonucleotide function
In general, oligonucleotides function by hybridizing to their complementary sequences. Used in many ways, oligos are particularly useful in studying gene expression and gene regulation mechanisms through PCR, microarray analysis, in situ hybridization, or biosensing applications. A good example of oligonucleotide use is as primers for PCR. Primers are single-stranded oligos required for initiation of DNA replication in PCR since DNA polymerases can only add nucleotides to existing nucleic acid sequence. During PCR, DNA polymerase attaches to primer-annealed target sequence in order to elongate the new DNA molecule. Since DNA is double-stranded, most PCR protocols require two primers, one forward and one reverse, to make a double-stranded product.
Oligonucleotides are also used as primers for DNA sequencing. Like PCR, conventional sequencing techniques use DNA polymerases that require existing DNA to initiate elongation. DNA oligos engineered to anneal specific target sequence are therefore used as primers for sequencing.
Other uses of oligonucleotides include construction of recombinant DNA and as molecular probes for use in techniques such as DNA microarrays, Southern and Northern blotting, antisense oligonucleotide (AS) analysis, and fluorescent in situ hybridization (FISH).
Techniques rely on the oligonucleotide properties of complementarity, sequence specificity, relative chemical stability, manipulability, and ease of labeling.
These techniques all rely on the oligonucleotide properties of complementarity, sequence specificity, relative chemical stability, manipulability, and ease of labeling. Complementarity, in particular, is key to the usefulness of oligos. Defined as the chemical recognition and specific hydrogen-bonding between nucleotide bases, complementarity drives the formation of double-stranded nucleic acid molecules and maintains the integrity of the sequence during replication and transcription. Complementary base pairs dictate sequence specificity and preserve sequence integrity by finding each other during the annealing, or hybridization, process to form double-stranded nucleic acid molecules, properties that make them valuable in techniques involving hybridization of target molecules.
Oligonucleotide use in gene expression studies
A fairly recent example of novel oligonucleotide use is as molecular barcode probes in gene expression profiling. Exemplified by NanoString’s nCounter® Pro Analysis System molecular barcode technology, these probes identify and quantify target transcripts in the absence of any enzymatic steps such as seen in PCR. Instead, an elegant hybridization protocol was developed to allow highly specific annealing in multiple types of samples for subsequent characterization.
Particularly useful in gene expression studies, the multipart probes used by nCounter Pro utilize DNA and RNA oligos.
Particularly useful in gene expression studies, the multipart probes used by nCounter Pro utilize DNA and RNA oligos. Based on unique 100 bp stretches of each target gene sequence, two separate 50mer DNA oligos are synthesized. One of the 50mer oligos is ligated to an additional DNA oligo to form a backbone complementary to a fluorescently labeled RNA molecule. The RNA oligo is labeled with a unique linear order of fluorescent colors to create a specific code for each gene of interest (Geiss). This DNA-RNA hybrid constitutes the reporter half of a probe pair, the second half of which consists of the second gene-specific 50mer oligo attached to 3’ repeats, forming the capture probe (Geiss). After the precise hybridization protocol is performed, the target RNA annealed to the reporter and capture probes creates a tripartite structure which is identified and quantified by the fluorescent signal of the reporter probe.

The predictable nature of oligonucleotide hybridization is sufficiently reliable to confer on the probes a high tolerance for challenging sample types, suboptimal RNA quality, and unequal copy numbers. nCounter Pro’s elegant fluorescent molecular barcoded probes demonstrate the priceless role of oligonucleotides in modern biotechnology.
References
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008 Mar;26(3):317-25. doi: 10.1038/nbt1385. Epub 2008 Feb 17. PMID: 18278033.

Related Content