Tips When Generating Single-Cell Spatial Data: Sample Selection
We are excited to share the second installment of tips for successful CosMx™ SMI single-cell spatial runs at 1000-plex. Our first installment of tips focused on data by tissue and disease type as well as tissue-specific sample preparation considerations; here we focus on evaluating tissue block quality, section quality, heterogeneity, and autofluorescence.
The CosMx™ SMI and decoder probes are not offered and/or delivered to the Federal Republic of Germany for use in the Federal Republic of Germany for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof in a method used in fluorescence in situ hybridization for detecting a plurality of analytes in a sample without the consent of the President and Fellows of Harvard College (Harvard Corporation) as owner of the German part of EP 2 794 928 B1. The use for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof is prohibited without the consent of the President and Fellows of Harvard College (Harvard Corporation).
Protocol Updates
Before we jump in to the second installment, we’d like to pass along two protocol changes that have demonstrated increased workflow performance. These changes have been included into the CosMx SMI Slide Preparation Manuals and the CosMx SMI Instrument Manual available in the NanoString University Document Library.
First, in the slide preparation workflow, the volume of Cell Segmentation Mix 1 (CD298/B2M) added by the user is decreased when preparing the antibody mixes for either the RNA or protein FFPE assays.
Table 1: Updated RNA Staining Mix Preparation
| Cell Segmentation Mix 1 (CD298/B2m) | Marker Mix 1* (Optional PanCK/CD45) | Marker Mix 2* (optional a la Carte*) | Blocking Buffer | Total Volume |
|---|---|---|---|---|
| 4 µL x n | 8 µL x n | 8 µL x n | 180 µL x n | 200 µL x n |
Table 2: Updated Protein Antibody Mix preparation
| Hs IO Protein Antibody Mix | CD298/B2M Segmentation Marker | PanCK/CD45 Marker (optional) | a la carte Marker (optional) | Diluted Custom Antibodies* (if applicable) | Buffer W | Total Volume |
|---|---|---|---|---|---|---|
| 62.5 µL x n | 2.5 µL x n | 5 µL x n | 5 µL x n | 1.25 µL x n | up to final volume of 125 µL x n | 125 µL x n |
Second, during instrument loading, the volume of pyranose oxidase added by the user to instrument Buffer 4 is decreased. This workflow change occurs during instrument loading and is on page 46 of the CosMx SMI Instrument Manual (for software v1.2) or page 51 of the CosMx SMI Instrument Manual (for software v1.3). Please refer to the manual for exact details.
NanoString recommends implementing these two improvements to your workflow moving forward.
Tips When Generating Single-Cell Spatial Data: Sample Selection
Tip 1: Evaluate Tissue Block Quality
Tissue block quality is a critical variable when generating single-cell spatial data. Two key factors when creating high-quality tissue blocks are fixation and ischemic time.
NanoString has validated protein and RNA tissues fixed with either 10% NBF or 4% PFA fixative. The use of other fixatives is generally not recommended. Because proper fixation is dependent on the diffusion of the fixative into the tissue, the recommended specimen thickness is 2-3 mm. At this thickness, most tissue types require a minimum fixation time of 24 hours at room temperature (up to 72 hours). Thicker tissue sections and tissue types such as bone, bloody or fatty tissues, HIV-containing tissues, and certain fetal tissues may require longer fixation times. Under-fixation of tissue can lead to degradation of the sample and poor morphological preservation. Over-fixation should also be avoided as it may result in non-specific background staining. The optimal fixation time for specimens may need to be empirically determined.
Ischemic time refers to the duration of time that a particular organ or tissue is deprived of adequate blood supply and consequently, oxygen and nutrients. While typically easier to control in mouse studies than human studies, ischemia is a critical factor in preventing tissue damage and RNA degradation. A shorter ischemic time is better for RNA quality.
NanoString recommends performing H&E staining to assess tissue block quality. The H&E stain can be used to determine if the tissue was properly fixed (Figure 1: Leica Biosystems, Process of Fixation and the Nature of Fixatives) as well as to note any areas of poor tissue quality, such as inconsistent thickness, tears, folds, and chatter (Figure 2: Leica Biosystems, H&E Basics Part 4: Troubleshooting H&E). This assessment can also be used to aid in Field of View (FOV) placement for serial sections when run on the CosMx SMI instrument.
Figure 1: H&E Staining to Determine Block Quality




Figure 2: Poor Tissue Handling or Sectioning Quality

Tip 2: Evaluate Tissue Section Quality
Tissue section quality plays a key role in signal generated from spatial assays. For CosMx RNA assays, it’s recommended to use mounted sections within two weeks for best results. Before sectioning, plan the placement of the tissue in the center of the slide’s scan area, indicated in green in Figure 3.
Figure 3: CosMx SMI tissue scan area

Successful completion of the downstream assay depends on technique during sectioning and slide preparation. Section adhesion and consistency of thickness have impact on downstream assay performance. For a more comprehensive guide on sectioning, please refer to Sample Sectioning Tips and Tricks for CosMx SMI and GeoMx DSP Experiments available on NanoString University.
Beginning with flat, well-mounted sections is important to maintain tissue integrity. Figure 4 shows the difference between high-quality and low-quality FFPE sections. The section shown on the left has few flaws and performed well during CosMx SMI workflow. The section on the right contains many tears and wrinkles, which can result in tissue detachment from the slide; this tissue section performed poorly during the sample preparation workflow.
Figure 4: Good and Bad Sections

There are numerous potential artifacts during FFPE sectioning. Most solid organs should be placed on ice for 30 minutes or longer prior to sectioning. However, it’s important to note that tissues, such as spleen and liver, being more delicate, should spend less than 30 minutes on ice. Sections should be cut at 5 μm thickness on a calibrated microtome and mounted in the center of the scan area (Figure 3). It is essential to always discard the first few sections from the block face. For a comprehensive guide, consult Leica Biosystems Introduction to Microtomy: Preparing & Sectioning Paraffin Embedded Tissue.
For frozen specimens, cryo-sections should be cut at 5-10 μm thickness on a calibrated cryostat and mounted immediately to the center of the scan area (Figure 3). During sectioning, it is important to cut across the tissue with a smooth, consistent turn of the hand wheel. Knife edge temperature has a large impact on section quality. See Table 3 for knife edge temperature recommendations. For a more comprehensive guide, please consult A Method for Preparation of Frozen Sections.
Table 3: Knife edge temperature for cryo sectioning
| Knife Temperature | Tissues |
|---|---|
| -10°C to -15°C | Adrenals, bone marrow, brain, cartilage, spleen, bloody tissue, testicular tissue |
| -15°C to -17°C | Bladder |
| -15°C to -18°C | Breast (less fatty), cervix, intestine, liver, thyroid |
| -15°C to -20°C | Kidney, lung, pancreas, prostate, ovary, rectal, skin without fat, uterus |
| -18°C to -22°C | Heart and vessel |
| -20°C to -25°C | Muscular |
| -25°C to -30°C | Skin with fat, breast (fatty) |
Tip 3: Consider Tissue Heterogeneity and Autofluorescence
As mentioned earlier, we recommend H&E staining with a serial section prior to beginning a CosMx SMI experiment. This serial section can be used to both evaluate tissue block quality and guide for FOV placement, an important part of experimental planning. By taking the time to properly consider and plan FOV placement, we can avoid selecting low-quality tissue areas and reduce instrument run time.
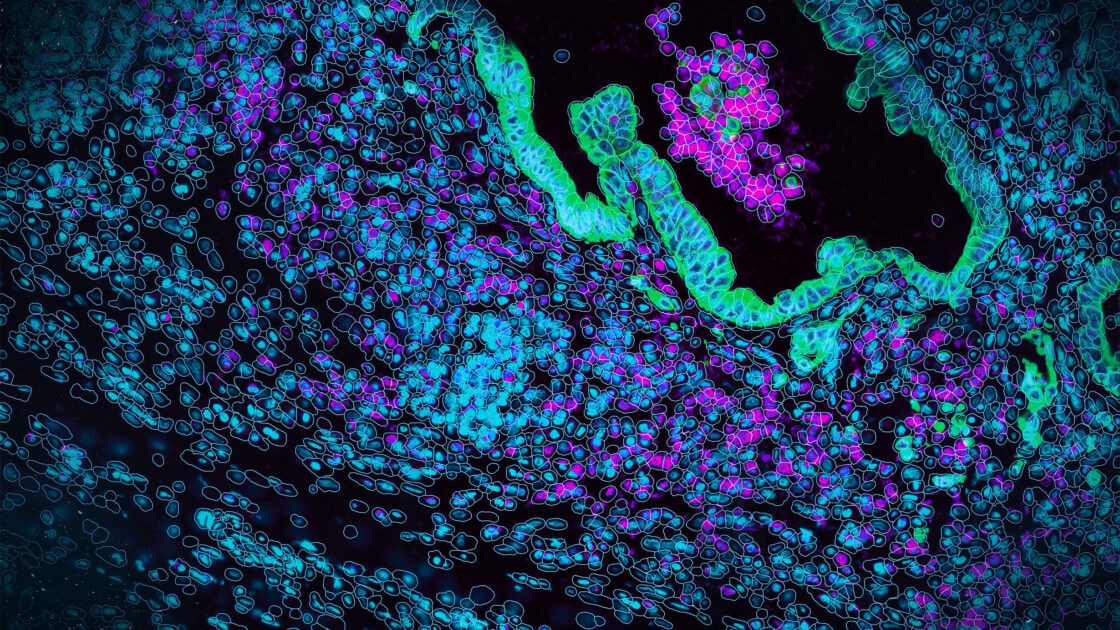
Consider the scientific question when selecting tissue areas. For example, the focus of the study below (Figure 5) was to study cancer, muscle, and normal tissue but not connective tissue. FOVs were placed to answer the scientific question of the study. On Figure 5, the left image shows the H&E section that was used to guide FOV placement for the serial section on the right that was run using the CosMx SMI 1K RNA assay. Also overlaid on the image on the right are the RNA counts per FOV. RNA counts will vary based on tissue morphology. Relatively lower RNA counts are typically observed in connective tissue, fatty tissue, and mucus.
Figure 5

Figure 6: Areas to avoid during FOV placement

Tissue autofluorescence is another variable to consider. High tissue autofluorescence can result in higher background and impact cell segmentation. Selecting the correct pre-bleaching profile (Table 4) during run set-up will minimize autofluorescence and improve data quality. If a tissue type of interest is not listed in the table below, start with the default condition (Configuration C) and adjust as needed. For reference, pre-bleaching times associated with each configuration are listed below (Table 4).
Table 4: Pre-bleaching profiles
| Tissue Type | RNA – Normal | RNA – Malignant | Protein |
|---|---|---|---|
| Brain | Configuration B | Configuration B | Configuration C |
| Skin | Configuration C | Configuration C | Configuration C |
| Lung | Configuration C | Configuration C | Configuration C |
| Breast | Configuration C | Configuration C | Configuration C |
| Liver | Configuration B | Configuration C | Configuration C |
| Colorectal | Configuration C | Configuration C | Configuration C |
| Tonsil | Configuration C | Configuration C | Configuration C |
| Pancreas | Configuration C | Configuration C | Configuration C |
| Kidney | Configuration B | Configuration B | Configuration C |
| Fresh Frozen | Configuration C | Configuration C | Configuration C |
| CPA | Configuration A | Configuration A | Configuration A |
When increasing pre-bleaching time (e.g. choosing Configuration B over C for tissues with higher autofluorescence), please note that the instrument will run for a longer duration (Table 5). For details on operating the CosMx SMI instrument, please refer to CosMx SMI Instrument User Manual.
Table 5: Pre-bleaching configuration impact on turnaround time
| Pre-bleaching Configuration Impact on TAT/Throughput | Configuration A 30 seconds | Configuration B 90 seconds | Configuration C 60 seconds |
| Pre-bleach duration 2 flow-cells 766 Total FOVs | 6.4 hours | 19.2 hours (12.8 hour increase) | 12.8 hours (6.4 hour increase) |
When placing FOVs, it is also important to consider tissue and cell-specific fluorescence. Table 6 highlights some of the various tissue and cell types to be aware of during FOV selection.
Table 6: Tissue/cell-specific autofluorescence considerations
| Tissue/Cell Type | Special Considerations |
|---|---|
| Blood Cells | Various components of blood, including red blood cells (erythrocytes) and white blood cells (leukocytes), can exhibit autofluorescence, primarily due to their hemoglobin content and other intracellular molecules. |
| Liver Tissue | Hepatocytes in the liver can produce autofluorescence, attributed to their content of flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NADH). |
| Nervous Tissue | Neurons and glial cells in the nervous system may display autofluorescence because of their mitochondria and other cellular components. |
| Lung Tissue | Lung tissue can be auto fluorescent, particularly due to the presence of collagen, elastin, and various proteins within the alveoli. |
| Kidney Tissue | Certain structures within the kidney, such as the glomerulus and renal tubules, may exhibit autofluorescence due to their constituent molecules. |
| Bone Tissue | Bone tissue, including bone matrix and osteocytes, can generate autofluorescence because of the presence of collagen and minerals like calcium. |
| Pancreatic Islets | Islets of Langerhans in the pancreas can show autofluorescence, primarily due to the presence of insulin-containing beta cells. |
| Intestinal Epithelium | The epithelial lining of the intestine can produce autofluorescence, potentially linked to the presence of mucins. |
| Placenta | Contains more red blood cells – see Blood Cells above. |
| Lipofuscins | Prominent in neurons, glial cells, and cardiac muscle cells, but found in a wide range of cell types, and predominately in post-mitotic cells. Lipofuscin has an enigmatic chemistry and stains positive for proteins, carbohydrates, and lipids, appearing brown in color. It usually occurs as small, punctate intracellular structures that are strongly fluorescent under any excitation ranging from 360 nm to 647 nm. |
| Elastin and Collagen | Typically, from blood vessel walls, elastic and collagen contain naturally fluorescent molecules that can be intensely fluorescent over a range of excitation wavelengths. |
Thank you for choosing CosMx SMI. If you have any further questions or need assistance, contact NanoString Support.




