Common questions in molecular biology: Are DNA and RNA oligonucleotides?
The short answer to whether DNA and RNA are oligonucleotides is yes. Oligonucleotides are short (oligo-) polymers of nucleotides, the basic subunit of nucleic acids such as DNA and RNA. Whether naturally occurring or synthetic, oligonucleotides base pair with targeted nucleic acids such as genomic DNA or any of the various cellular RNAs. Relatively small (typically 12–25 base pairs), oligonucleotides (also known as oligomers or oligos) may be chemically modified or unmodified for use in an extensive range of molecular genetics applications.
Functions of oligonucleotides
In nature, oligonucleotides exist mainly as small non-coding RNAs which generally work by hybridizing to their complementary sequences. The complementary (or antisense) sequence of these small regulatory RNAs usually targets mRNAs to modify protein production by means of such functions as RNA editing, gene regulation, and RNA interference. Non-coding RNAs include small nuclear RNA (snRNA), microRNA (miRNA), and small interfering RNA (siRNA; Wang and Farhana).
Proven effective against diseases such as Duchenne muscular dystrophy and spinal muscular atrophy, antisense therapy has received approval for use in several countries.
The concept of antisense oligonucleotide (ASO) interference with mRNA function has recently been applied to targeting disease processes. Known as antisense therapy, ASOs are used to alter disease-related mRNA expression through mechanisms such as ribonuclease-mediated decay of pre-mRNA, direct steric blockage, and exon sequence modulation by binding to pre-mRNA splice sites. Proven effective against diseases such as Duchenne muscular dystrophy and spinal muscular atrophy, among others, antisense therapy using ASOs has received approval for use in several countries (Kim).
Oligonucleotides such as RNA ASOs and many DNA ones are commonly synthesized using solid-phase chemistry. Used in many different ways, synthetic oligos function as primers in PCR and as probes in microarray analysis, in situ hybridization, and biosensing applications.
Oligonucleotide-based technologies continue to increase as knowledge regarding gene expression and gene regulation mechanisms expands exponentially. Especially important to the usefulness and versatility of oligonucleotides are their properties of complementarity and sequence specificity, as well as their relative chemical stability, manipulability, and ease of labeling. Complementarity, in particular, makes oligonucleotides invaluable, as it drives single-stranded nucleic acids to find each other, forming double-stranded molecules during the annealing process. Driven by specific hydrogen-bonding and chemical recognition between nucleotide bases — adenine pairs with thymine in DNA and uracil in RNA while guanine pairs with cytosine in DNA and RNA — complementarity maintains the integrity of DNA sequence by dictating sequence specificity, which in turn makes the annealing/hybridization process highly predictable.
Reliably predictable hybridization makes oligonucleotides valuable as probes and other molecular tools.
Using DNA and RNA oligonucleotides together
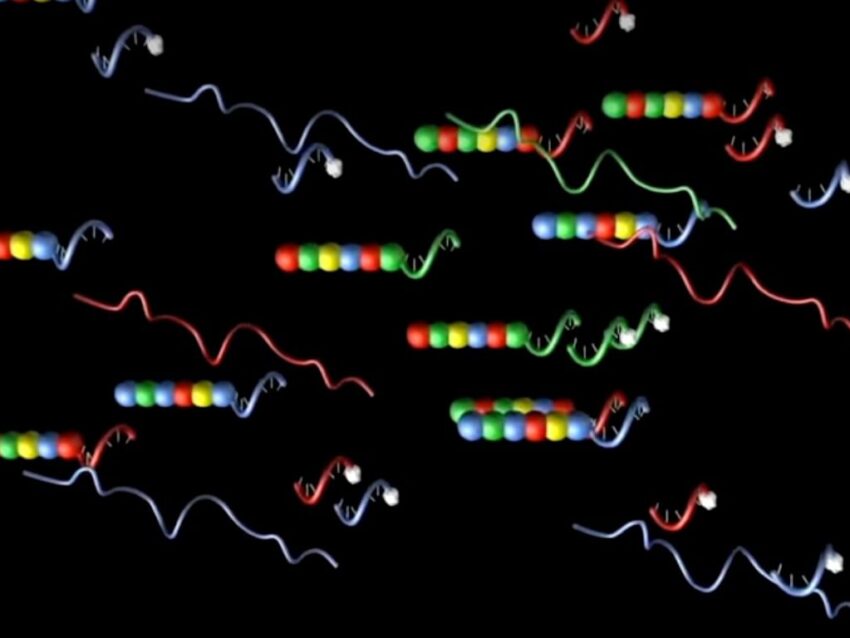
Predictable hybridization has enabled the development of technologies using both DNA and RNA oligos as probes. One in particular, pioneered by NanoString for use in the nCounter® Pro Analysis System, uses two DNA oligos and one RNA oligo to form multi-part probes for direct identification and quantitation during gene expression profiling in tissues.
The specifically-designed oligonucleotides constituting the heart of each nCounter probe are based on unique 100 bp stretches of each target gene sequence, forming two separate 50mer DNA oligos to span the region. One of the target 50mer oligos has an additional DNA oligo sequence ligated to one end to form the backbone, which is annealed to a complementary RNA molecule. Individual segments of the RNA oligo are fluorescently labeled with a unique order of colors to create a specific code for each gene of interest (Geiss). This DNA-RNA hybrid molecule constitutes the reporter half of the probe pair.
The elegance of nCounter Pro’s fluorescent molecular barcoded probes is a great demonstration of oligonucleotides’ priceless role in modern biotechnology.
The second half of the two-part probe consists of the second gene-specific sequence attached to 3’ repeats to form the capture probe (Geiss). A precise hybridization protocol anneals the target RNA to the reporter and capture probes, creating a tripartite structure which is identified and quantified by the specific fluorescent signal of the reporter probe. The specific predictable nature of oligonucleotides provides reliable hybridization of these probes, even conferring on them a high tolerance for challenging sample types, suboptimal RNA quality and unequal copy numbers. The elegance of nCounter Pro’s fluorescent molecular barcoded probes is a great demonstration of oligonucleotides’ priceless role in modern biotechnology.
References
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008 Mar;26(3):317-25. doi: 10.1038/nbt1385. Epub 2008 Feb 17. PMID: 18278033.
Kim YJ, Krainer AR. Antisense Oligonucleotide Therapeutics for Cystic Fibrosis: Recent Developments and Perspectives. Mol Cells. 2023 Jan 31;46(1):10-20. doi: 10.14348/molcells.2023.2172. Epub 2023 Jan 15. PMID: 36697233; PMCID: PMC9880599.
Wang D, Farhana A. Biochemistry, RNA Structure. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.

Related Content