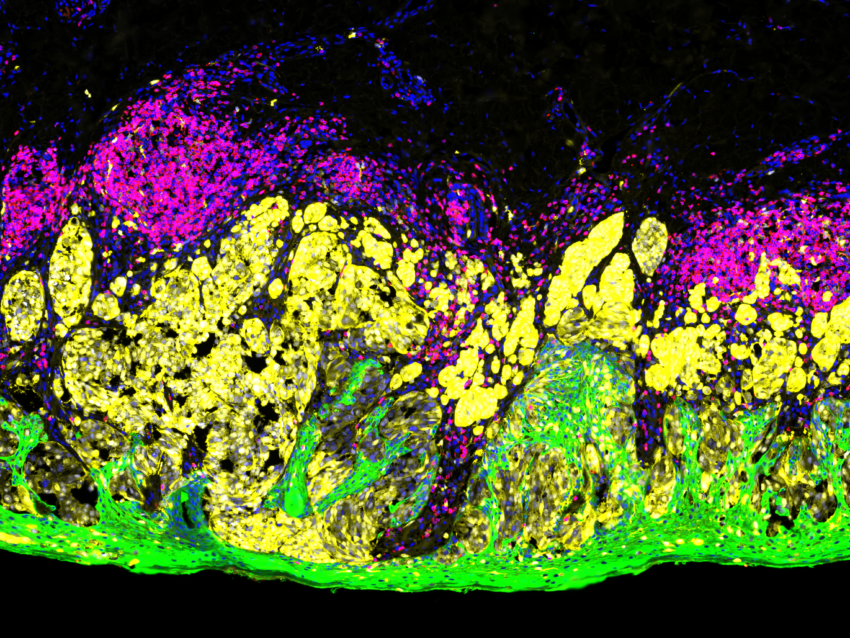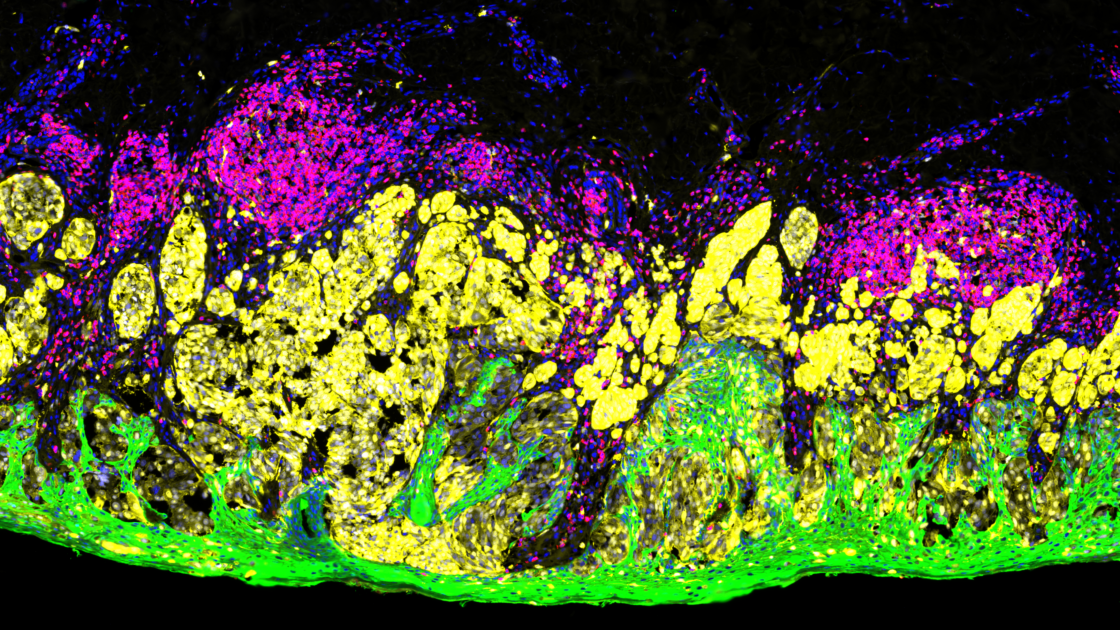
What is immunofluorescence used for?
Immunofluorescence techniques are being increasingly used as an imaging and diagnostic tool in clinical pathology, oncology, and infectious disease, particularly for the study of biomarkers related to cancer diagnosis, prognosis, and drug development.
Immunofluorescence methods are based on the principle of antigen-antibody binding where an antibody tagged with a fluorescent dye is used to visualize the localization and distribution of a specific antigen in cells or tissues. The bound antigen-antibody complex is then visualized using various microscopy techniques.
Some applications of immunofluorescence for biological research are antigen analysis in fresh, frozen, or fixed tissues such as formalin-fixed paraffin-embedded (FFPE) tissue sections, detection of circulating autoantibodies or immune cells, observation of parasitic or bacterial specimens, detection and localization of specific chromosomal DNA sequences, and defining the spatial-temporal patterns of gene expression within intact cells/tissues.
Multiplex immunofluorescence
Immunofluorescence can be multiplexed to detect more than one antigen at a time and has become a powerful supplement to technologies such as next-generation sequencing (NGS). Multiplex immunofluorescence of tissue is unique in its ability to provide both expression and location data for several antigens while preserving tissue architecture and spatial context. Several tissue-based multiplex immunofluorescence methods have been developed with different strategies for detection that can be broadly categorized as: stain removal technologies, fluorophore inactivation technologies, multiplexed signal amplification, mass cytometry, and DNA barcoding technologies.
Many of these multiplex immunofluorescence techniques were developed to be compatible with FFPE or fresh frozen tissues, thus opening doors to archival FFPE tissue and tissue from patient biopsies, a valuable resource for protein analysis. Today, many of the single marker immunofluorescence techniques are slowly being replaced by multiplex immunofluorescence assays used not only for diagnostic purposes but also for patient stratification or staging, treatment decisions, and prognosis.
The GeoMx® Digital Spatial Profiler
NanoString’s GeoMx® Digital Spatial Profiler (DSP) platform combines standard immunofluorescence techniques with oligonucleotide barcoding technology, enabling researchers to rapidly characterize tissue morphology while doing high-throughput, high-plex profiling of both RNA and protein. The GeoMx DSP is compatible to work on a variety of samples such as core needle biopsies, tissue microarrays, fresh frozen tissue, and FFPE tissue.
Among the available multiplex immunofluorescence techniques, the GeoMx DSP platform offers the unique advantage of providing a spatial quantitative measurement of both RNA and protein abundance in a defined region of interest (ROI) of a tissue section. The GeoMx DSP platform is a non-destructive method that preserves clinical samples, which are limited and precious, for secondary analysis with other approaches; only a single section is required.
There are multiple GeoMx assays available off-the-shelf with pre-validated antibodies or RNA probes for studying various diseases or pathways with the potential for customization. For example, the GeoMx Cancer Transcriptome Atlas panel allows for comprehensive spatial analysis of cancer-related pathways using the GeoMx DSP.
Applications of GeoMx DSP
The GeoMx DSP platform has been successfully used to address several biological questions in many research fields including oncology, immunology, neuroscience, and infectious disease.
Much of the research has been done using FFPE tissue making the GeoMx DSP suitable for translational and clinical research. Furthermore, the data generated by GeoMx DSP has been validated with independent methods, thereby giving confidence to the results from GeoMx studies. Many studies have shown the feasibility of using the GeoMx DSP platform to identify biomarkers in different settings, including predictive biomarkers for immunotherapy in different types of tumors. Immunoprofiling using the GeoMx DSP has helped unravel how immune cells can respond to different diseases and therapies.
The study by Toki et al. used the GeoMx DSP to identify biomarkers that were predictive for response to neoadjuvant therapy in patients with high-risk melanoma. The authors investigated specific cell populations within the tumor from pretreatment biopsies of patients with melanoma who received therapy. The GeoMx DSP enabled them to correlate clinical outcomes with the expression of 44 proteins in 3 independent spatially resolved compartments (macrophage, leukocyte, and melanocyte). They observed that programmed death ligand-1 (PD-L1) expression in the macrophage compartment, but not the tumor cell compartment, was predictive for an objective response, progression-free survival, and overall survival.1Toki MI, Merritt CR, Wong PF, Smithy JW, Kluger HM, Syrigos KN, Ong GT, Warren SE, Beechem JM, Rimm DL. High-Plex Predictive Marker Discovery for Melanoma Immunotherapy-Treated Patients Using Digital Spatial Profiling. Clin Cancer Res. 2019 Sep 15;25(18):5503-5512. doi: 10.1158/1078-0432.CCR-19-0104. Epub 2019 Jun 12. PMID: 31189645; PMCID: PMC6744974.
Another study by Moutafi et al. investigated patients with advanced non-small-cell lung cancer (NSCLC) who failed to derive significant benefit from PD-1 axis blockade therapy, a standard treatment for multiple malignancies. Using the GeoMx DSP, it was shown that CD44, a non-kinase transmembrane glycoprotein, was overexpressed in the tumor compartment, but not in the immune compartment, predicting clinical outcomes from PD-1 axis blockade.2Moutafi MK, Molero M, Martinez Morilla S, Baena J, Vathiotis IA, Gavrielatou N, Castro-Labrador L, de Garibay GR, Adradas V, Orive D, Valencia K, Calvo A, Montuenga LM, Ponce Aix S, Schalper KA, Herbst RS, Paz-Ares L, Rimm DL, Zugazagoitia J. Spatially resolved proteomic profiling identifies tumor cell CD44 as a biomarker associated with sensitivity to PD-1 axis blockade in advanced non-small-cell lung cancer. J Immunother Cancer. 2022 Aug;10(8):e004757. doi: 10.1136/jitc-2022-004757. PMID: 36002182; PMCID: PMC9413286.
References
- 1Toki MI, Merritt CR, Wong PF, Smithy JW, Kluger HM, Syrigos KN, Ong GT, Warren SE, Beechem JM, Rimm DL. High-Plex Predictive Marker Discovery for Melanoma Immunotherapy-Treated Patients Using Digital Spatial Profiling. Clin Cancer Res. 2019 Sep 15;25(18):5503-5512. doi: 10.1158/1078-0432.CCR-19-0104. Epub 2019 Jun 12. PMID: 31189645; PMCID: PMC6744974.
- 2Moutafi MK, Molero M, Martinez Morilla S, Baena J, Vathiotis IA, Gavrielatou N, Castro-Labrador L, de Garibay GR, Adradas V, Orive D, Valencia K, Calvo A, Montuenga LM, Ponce Aix S, Schalper KA, Herbst RS, Paz-Ares L, Rimm DL, Zugazagoitia J. Spatially resolved proteomic profiling identifies tumor cell CD44 as a biomarker associated with sensitivity to PD-1 axis blockade in advanced non-small-cell lung cancer. J Immunother Cancer. 2022 Aug;10(8):e004757. doi: 10.1136/jitc-2022-004757. PMID: 36002182; PMCID: PMC9413286.