What is Regenerative Medicine?
Regenerative medicine harnesses tissue renewal’s ability to develop methods for repairing, replacing, and regenerating damaged tissues.
What are examples of regenerative medicine?
Regenerative medicine uses gene therapy, tissue engineering, transplant, and stem cell biology. Several critical illnesses are already treated with stem cells: hematological stem cell transplant treats hematological disorders and cancers, and mesenchymal stem cells are used to treat cartilage and bone diseases.
Regeneration or tissue renewal is indeed an inherent characteristic of several mammalian tissues, such as epithelia and blood cells. In these tissues, multipotent stem cells can self-renew or differentiate into multiple but limited cell types: old cells are shed and replaced by new cells through tightly regulated signaling circuits (1).
Pluripotent stem cells are the new frontier of regenerative medicine: they can self-renew while maintaining their pluripotent status until they are induced to differentiate into any cell type. These characteristics can potentially treat otherwise deadly or debilitating diseases, as evidenced by the plethora of NIH-funded clinical trials.
For clinical use, pluripotent stem cells must be generated, maintained, and expanded without triggering their natural inclination to differentiate into specific cell types. Continue reading to find out how they are made.
What are the sources of Pluripotent Stem Cells?
Fundamentally, there are two sources of pluripotent stem cells: embryonic-derived (ESCs) and tissue-derived (iPSCs) (2). Their integral characteristic is the ability to indefinitely self-renew without differentiating into a specific cell type.
Embryonic stem cells don’t need to be produced as they derive from embryos 4-5 days after fertilization and before implantation when the cells – usually between 50 and 150 – are still pluripotent and able to develop into all tissue types.
Embryonic stem cells are challenging to maintain for clinical use for several reasons: they can form teratomas – tumor-like growths with altered cell differentiation patterns – as several rounds of replication can result in genetic errors, they are hard to produce in large-scale within Good Manufacturing Practice (GMP) accredited facilities, and they are ethically debatable.
Induced pluripotent stem cells (iPSC) have similar properties to ESCs but are generated from adult somatic cells upon re-activation of 4 transcription factors, known as the OSKM factors (OCT4, SOX2, KLF4 and MYC). These factors can induce somatic cells to “turn back the clock” and return to their pluripotent status.
iPSC bypass ethical considerations since they are derived from adult patients, with the added advantage of allowing iPSCs to be genetically matched with a recipient: pluripotent stem cells from compatible donors can be transplanted. However, for their own pluripotent nature, they remain teratogenic.
Strategies for modulating the regulatory signaling pathways that promote and maintain pluripotency while minimizing the risks of genetic aberrations are under study.
Let’s look at some of these strategies.
Strategies in Regenerative Medicine: Stem Cells Generation and Self-Renewal
Regulation of stem cell self-renewal requires a complicated network of regulatory signaling pathways and external influences to regulate proliferation potential, inhibit apoptosis/cell death, and block differentiation. These pathways can promote pluripotency by either directly suppressing differentiation-promoting genes or by enhancing self-renewal transcriptional pathways.
Induced PSCs require a different strategy because they must be generated: the first step is to reprogram an adult, differentiated cell into its pluripotent state. In 2006, Yamanaka and colleagues published their discovery of a minimal network of transcription factors, the OKSM factors. These factors could be used to reprogram fibroblasts in pluripotent stem cells capable of differentiating into the three embryonic layers and therefore in all tissue types (3).
These external transcription factors need to be delivered to the cell by a vector, and there are different ways to do it.
Virus-based delivery systems have been the mainstream method of generating iPSCs. These vectors can be integrating or non-integrating. In integrating vectors, the DNA content of the vectors translocates to the nucleus where it integrates with the host cell’s genome. But genomic integrations are potentially tumorigenic, and the product can harbor genetic abnormalities. Thus, iPSC generation methods that don’t require integration of a viral vector are highly desirable as the genetic material of non-integrating vectors remains in the cytoplasm.
Non-integrating methods of reprogramming can use either RNA transfection – where cells are transfected with in vitro–transcribed mRNAs encoding the necessary transcription factors – or by using an RNA virus like the Sendai virus that replicates only in the cytoplasm (4).
Pathways to Maintaining Pluripotency
Maintaining pluripotency means that the stem cell can replicate without differentiating. Conceptually, signaling pathways maintain pluripotency by either inhibiting differentiation-promoting genes or by directly supporting self-renewal. There are multiple pathways under study, but the following is probably the most well-known.
In human embryonic stem cells, the transcription factors responsible for maintaining the pluripotent state are POU5F1, NANOG, and SOX2. They are thought to be the master regulators as they influence the molecules promoting self-renewal. They also silence other developmental factors that are active during lineage commitment and cellular differentiation (5)
The other critical component of the self-renewal machinery is TCF3, a component of the Wnt pathway that in stem cells controls cell fate specification, cell proliferation, and cell migration, processes necessary for normal formation of tissues. TCF3 associates with OCT4 and NANOG. Thus, TCF3 and the Wnt pathway are integral to the core regulatory circuitry of stem cells (6).
Strategies in Regenerative Medicine: Pluripotent Stem Cells Expansion
To be studied or used in a clinical setting, stem cells need to be grown. Therefore, another significant challenge in cell-based regenerative medicine is stem cell expansion. Clinical applications of human pluripotent stem cells require large cell quantities that cannot be produced by conventional adherent culture techniques (7). The approach employed needs to be scalable and usable in good manufacturing practice (GMP) accredited facilities.
Conventional expansion methods use feeder layers or feeder-free systems.
Feeder layer systems are systems where a layer of cells unable to replicate secretes the growth factors the stem cells need for self-renewal and proliferation.
Feeder-free systems use media enriched with growth factors that regulate pluripotency-associated genes. In these systems, stem cells are cultured upon a layer of extracellular matrix proteins, such as fibronectin, laminin, or other industrial-made matrix products.
These conventional methods are low cost, widely used, and well documented. This makes them ideal for research labs. However, these methods are limited by the high costs of scaling up for GMP-cleared facilities, possible variations across batches, and the use of foreign components (which are undesirable in the clinical setting.).
Non-conventional expansion methods overcome these limitations. They can be 2-dimensional (2D) or 3-dimensional (3D) and take into consideration the fact that stem cells’ self-renewal is dependent upon their mechanical microenvironment. 3D culture methods effectively mimic tissue architecture by re-creating the in vivo cells and matrix interactions by employing matrices or scaffolds, in addition to the cells, to support more complex structures (7).
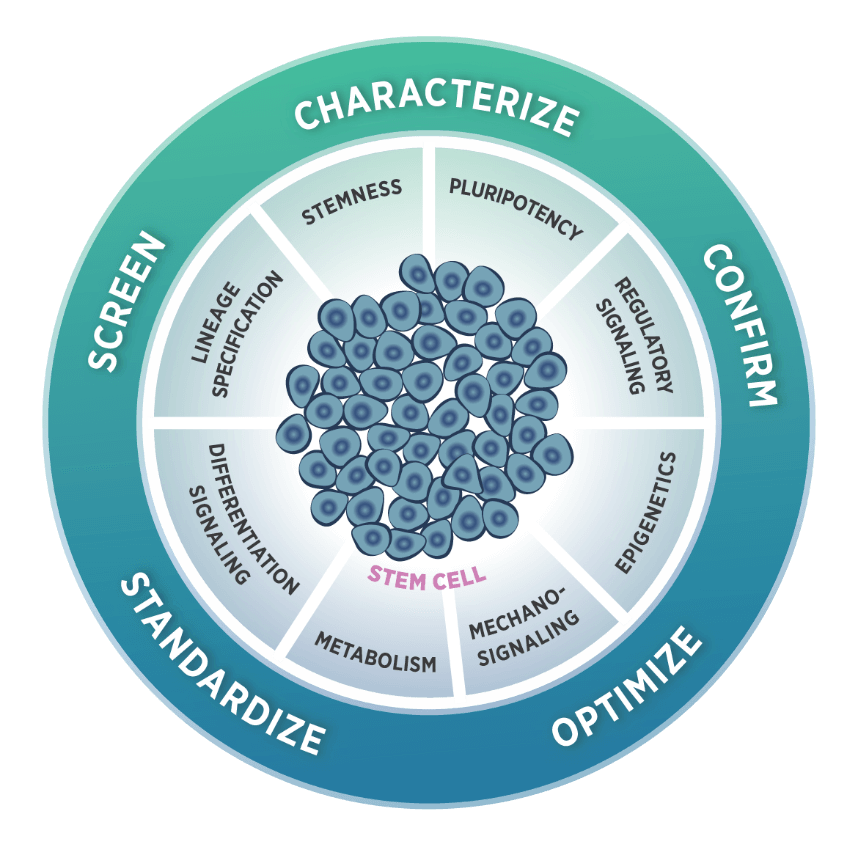
The Stem Cells and Regenerative Medicine Panel
NanoString gene expression panels have supported regenerative medicine, first with the Neuroscience Panels, followed by the Human Organ Transplant Panel. In 2021, our nCounter® Stem Cell Characterization Panel was launched to focus directly on the biology of self-renewal, expansion, differentiation, and culture of pluripotent stem cells. With this panel, scientists can monitor stemness by measuring proliferation, cell cycle, apoptosis, and senescence. They can monitor pluripotency through markers, regulatory pathways, epigenetics, and mechano-signaling. The 770 probes included in the panel cover all relevant content in pluripotent stem cell research, including 12 internal reference genes for data normalization. This panel was designed to support stem cell research on the nCounter® platform with our cost-effective, robust, and straightforward solution for gene expression analysis. Looking forward, the DSP platform can also be used to explore organoid tissue function. For example, the Whole Transcriptome Assay (WTA) detects over 18,000 genes in a single reaction. This technology can be used to study drug response by reading the gene expression profile differences on different regions of the organoid, comparing between various cell types or layers of tissue.
References
- https://www.nature.com/articles/cr2012172
- https://www.nature.com/subjects/pluripotent-stem-cells
- https://pubmed.ncbi.nlm.nih.gov/16904174/
- A comparison of non-integrating reprogramming methods | Nature Biotechnology
- Core Transcriptional Regulatory Circuitry in Human Embryonic Stem Cells (nih.gov)
- Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells (cshlp.org)
- Frontiers | Emerging Methods for Enhancing Pluripotent Stem Cell Expansion | Cell and Developmental Biology (frontiersin.org)
For Research Use Only. Not for use in diagnostic procedures.

