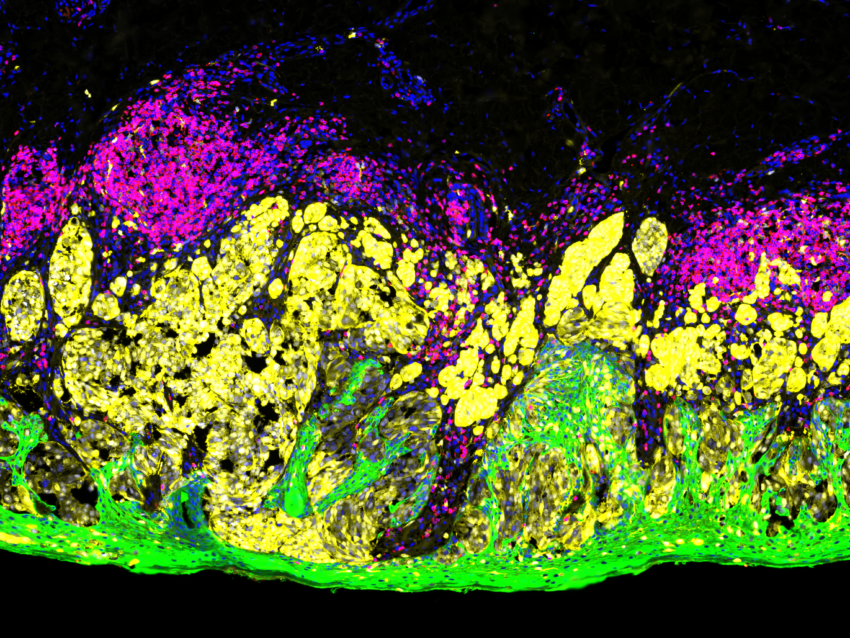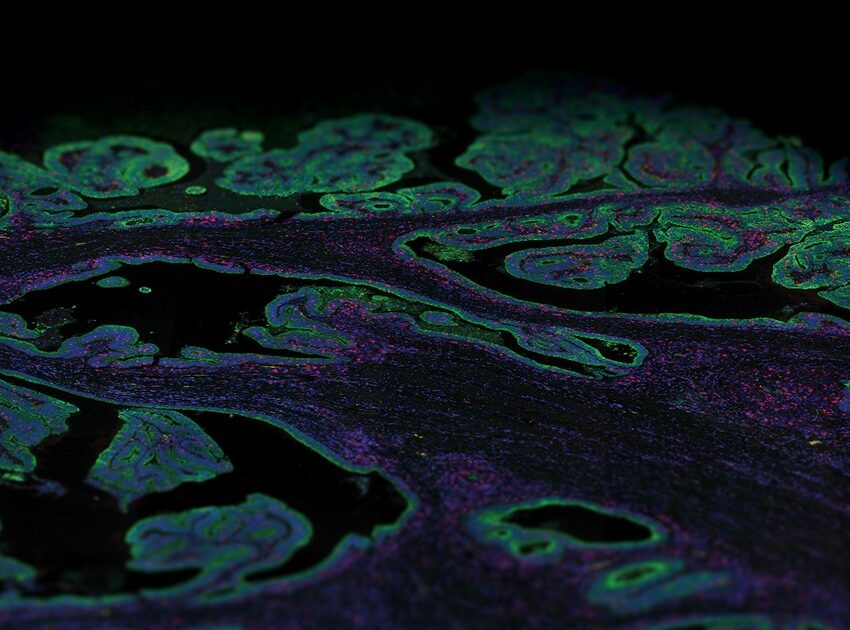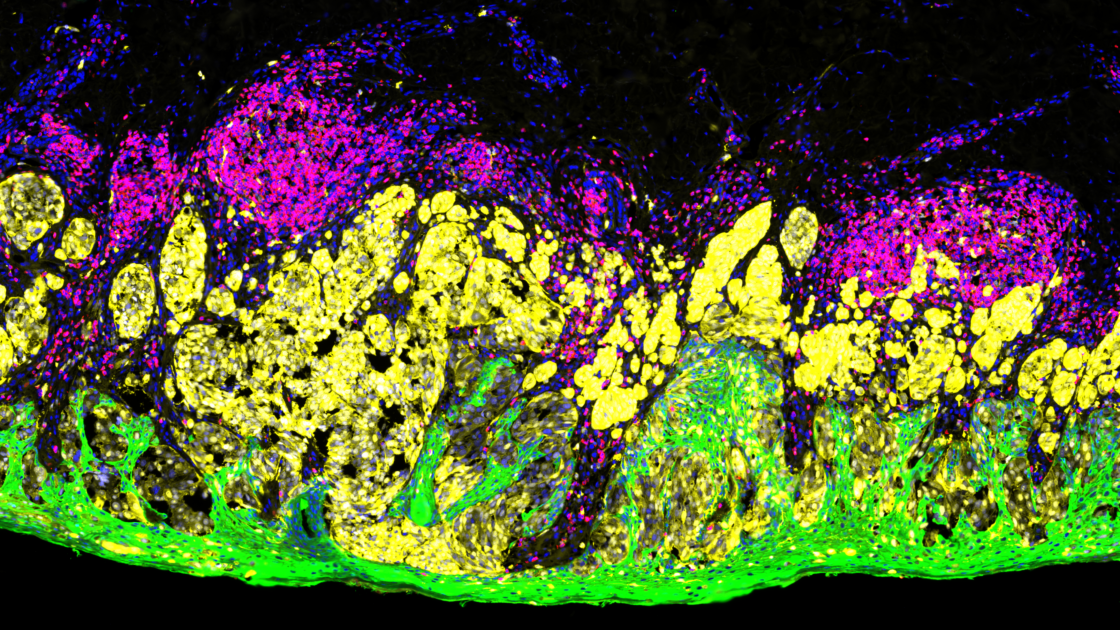
What is the principle of immunofluorescence?
Immunofluorescence staining was first used in 1942 and has since remained a highly reliable and powerful technique for a wide range of research and diagnostics.
The principle of immunofluorescence is based on antigen-antibody binding using fluorescent detection. Various antigens such as proteins, glycans, and small biological and non-biological molecules are detected and visualized within cells or tissues using fluorophores. Fluorophores are compounds that emit light when exposed to a certain wavelength of light. Thus, various combinations of specific antibodies tagged with fluorophores allow the visualization of different types of molecules in any given tissue or cell suspension. Imaging of tissue sections is generally done with a fluorescent microscope or confocal microscope, depending on the biological question being addressed by this method.
Immunofluorescence is a simple and straightforward biological tool and therefore its applications in research and patient care are numerous.
What are the different types of immunofluorescence staining?
The detection of antigens by immunofluorescence can be done either by the direct or indirect method.
In the direct immunofluorescence method, the antibody is directly conjugated with a fluorophore and visualized after the conjugated antibody binds the antigen of interest. The direct immunofluorescence method is particularly useful for rapid diagnosis such as the detection of bacterial/viral infections, suspected autoimmune disease, and connective tissue disease.
Detection of antigens via indirect immunofluorescence method is a two-step process. The first step involves binding the primary antibody to the antigen of interest after which a secondary antibody conjugated to a fluorophore is used to detect the primary antibody. Thus, the indirect method allows for high sensitivity as several secondary antibodies can bind to a single primary antibody. The indirect immunofluorescence method is widely used in clinical settings to detect low-abundance proteins or circulating autoantibodies, for example, samples from patients with systemic lupus erythematosus who have increased levels of circulating anti-nuclear autoantibodies against both DNA and various proteins that bind to DNA.
The advantages of immunofluorescence
Immunofluorescent methods are a simple yet effective method for determining the presence of different biological molecules such as proteins, carbohydrates, and small molecules.
Immunofluorescence methods offer enhanced sensitivity, and can simultaneously detect multiple epitopes (multiplexing).
There are several advantages to immunofluorescence compared to traditional immunohistochemistry. Immunofluorescence methods offer enhanced sensitivity by increasing the number of fluorophores per primary antibody molecule, allowing the generation of quantitative data. Furthermore, immunofluorescence methods have been developed that can simultaneously detect multiple epitopes (multiplexing). Currently, the use of immunofluorescence in routine diagnostic pathology is limited to in situ hybridization (ISH) to detect chromosomal abnormalities and to antibody-based investigation of specific immune and genetic diseases in dermatopathology and nephropathology. But the development of precision medicine guided targeted therapy, particularly immunotherapy, requires the identification of biomarkers that can predict therapy efficacy and signaling pathways that help understand cell behavior; therefore, multiplex immunofluorescence has become an essential tool for analysis.
Multiplex immunofluorescence
Multiplex immunofluorescence technologies use fluorescence emission as a means of marker visualization with some combination of simultaneous and or cyclic sequential labeling and detection and include the use of haptens, cyclic tyramide-based amplification, and oligonucleotide barcodes.

NanoSting’s GeoMx® Digital Spatial Profiler combines conventional fluorescence imaging with barcoded antibodies or in situ hybridization probes for doing high-throughput and high-plex profiling of both RNA and protein across various tissue sections, particularly FFPE tissue sections. The barcodes act as identifiers for spatial position and are counted using either the nCounter® Analysis System or next-generation sequencing (NGS).
The more recently introduced single cell CosMx™ Spatial Molecular Imager compliments the GeoMx DSP and uses a combination of sensitive cyclic ISH chemistry and an ultra-high-resolution imaging readout to spatially profile proteins and RNA in tissue sections with single cell and subcellular resolution. Used together, the GeoMx DSP and CosMx SMI can provide insights for cell atlasing, tissue phenotyping, analysis of cell-cell interactions and celullar state, pathway analysis, and biomarker discovery and validation.