What are immunofluorescence techniques?
Tissue and cell samples constitute the main source of samples for protein or RNA expression analysis in biology and help us understand how cells interact, organize themselves, and drive various biological functions. Immunofluorescence techniques that rely on the basic principle of antigen-antibody binding with the use of fluorescent tags have become an essential method for translational and basic research.
Immunofluorescence techniques have been further developed over the last decade to work well with samples that are preserved by formalin fixation and paraffin-embedment, a routine form of tissue preservation in clinical practice. It is estimated that there are 400 million to more than a billion formalin-fixed, paraffin-embedded (FFPE) samples in hospitals and tissue banks worldwide. Thus, FFPE tissue samples are a valuable resource and have opened doors to retrospective analyses for experimental research, diagnostic/drug development, and biomarker identification and validation.
Techniques that use immunofluorescence
Immunofluorescence technology is constantly evolving to increase specificity, sensitivity, and throughput using advanced labeling as well as different detection strategies. Purified antibodies such as monoclonal or polyclonal antibodies are most often used to identify and locate intracellular and extracellular proteins in cell cultures or intact tissue sections. Other advancements in immunofluorescence techniques include flow cytometry which utilizes a laser beam and light detector to quantify antigens or antibodies on single intact cells in suspension.
Fluorescence in situ hybridization (FISH) is another powerful tool to visualize target DNA sequences or messenger RNA (mRNA) transcripts in cultured cells or tissue sections. The method of single molecule FISH (smFISH) is a further improvement of the FISH technique that uses multiple probes with the sensitivity to resolve individual mRNA transcripts. A large volume of data concerning mRNA and protein expression is now available and — along with the advent of NGS methods and microfluidics — is rapidly enhancing our understanding of cellular organization and tissue structure with only a small amount of sample.
Multiplex immunofluorescence
Although conventional immunofluorescence is a widely used diagnostic technique in tissue pathology, limitations such as high inter‐observer variability and the capacity to label only one marker per tissue section prevent rapid and high-plex analysis. To circumvent these constraints, the emergence of multiplex immunofluorescence technology has allowed the simultaneous detection of multiple markers simultaneously from a single tissue section.
High‐throughput multiplex staining and quantitative analysis with spatial resolution is now possible with evolving technologies that are efficient, cost‐effective, and easily adaptable to both research and clinical settings.
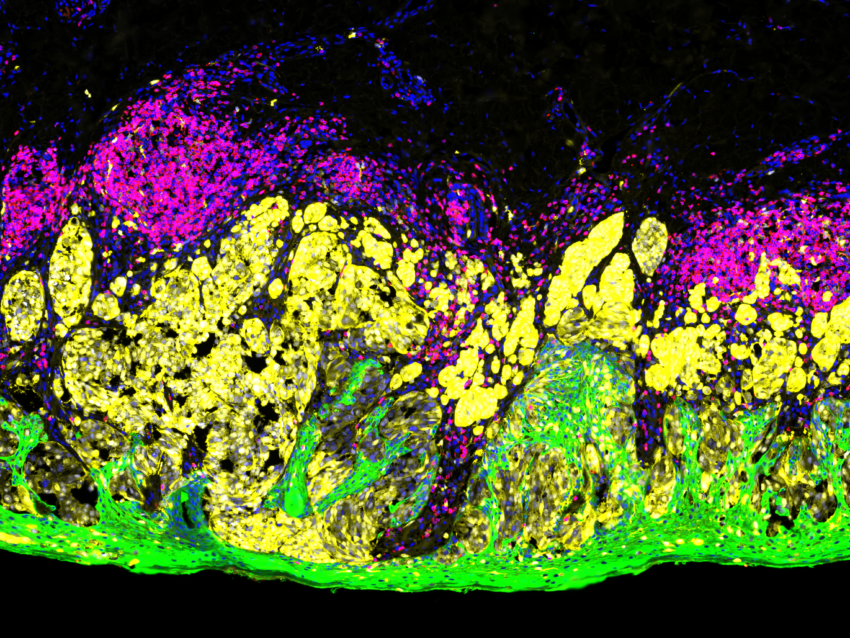
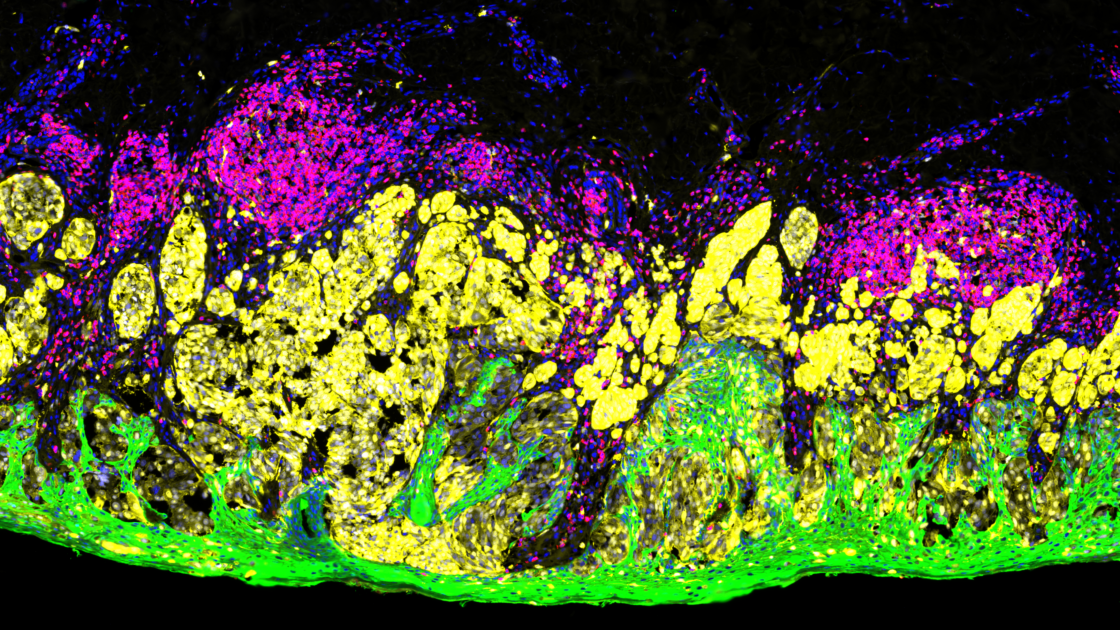
Multiplex immunofluorescence provides a comprehensive study of cell composition, cellular functionality, and cell‐cell interactions. High‐throughput multiplex staining and quantitative analysis with spatial resolution is now possible with evolving technologies that are efficient, cost‐effective, and easily adaptable to both research and clinical settings. A wide variety of multiplexed immunofluorescence imaging technologies based on techniques such as cyclic immunofluorescence, tyramide‐based immunofluorescence, epitope‐targeted mass spectrometry, and RNA detection have been developed. As an example, the multiplex spatial multiomics technologies of NanoString’s GeoMx® Digital Spatial Profiler and CosMx™ Spatial Molecular Imager (SMI) technologies are described below.
The GeoMx® Digital Spatial Profiler
The GeoMx DSP system developed by NanoString Technologies is an example of a multiplex tissue imaging and spatial multiomics platform that combines standard immunofluorescence techniques with oligonucleotide barcoding technology to enable researchers to rapidly characterize tissue morphology while spatially profiling RNA and protein on various sample types, particularly FFPE tissue sections.

The GeoMx DSP technologies use molecular barcoded oligonucleotide tags that are conjugated to antibodies or RNA probes antibodies using a photocleavable UV light‐sensitive linker. These labeled probes are then hybridized onto tissue sections that are also stained with fluorescent morphological markers that identify tissue anatomy and landscape. The morphological features identified thus help in the selection of a region to be analyzed. The selected region is then excited with UV light, triggering the release of the oligonucleotide tags which are retrieved from the surface of the tissue, enabling the sample to be reused. The oligonucleotide tags then undergo quantitative analysis using NGS and are mapped back to tissue location to allow spatial profiling at the selected region of interest.
The CosMx™ Spatial Molecular Imager
The more recent CosMx™ SMI complements the GeoMx DSP and uses a combination of sensitive cyclic in situ hybridization chemistry and an ultra-high-resolution imaging readout instrument. The CosMx SMI platform profiles both mRNA transcripts or protein levels to a single cell and subcellular resolution across the entire tissue section.
CosMx system is ideal to explore questions downstream of whole transcriptome analysis carried out with GeoMx, as these two systems can be used for complementary experiments in the same scientific program.
Together, GeoMx DSP and CosMx SMI can be applied to a number of techniques such as the generation of cell/tissue atlas, tissue phenotyping, or investigating questions in biology such as studying cell-cell interactions, cellular processes, and biomarker discovery.
The CosMx™ SMI and decoder probes are not offered and/or delivered to the Federal Republic of Germany for use in the Federal Republic of Germany for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof in a method used in fluorescence in situ hybridization for detecting a plurality of analytes in a sample without the consent of the President and Fellows of Harvard College (Harvard Corporation) as owner of the German part of EP 2 794 928 B1. The use for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof is prohibited without the consent of the President and Fellows of Harvard College (Harvard Corporation).